Nayzilam
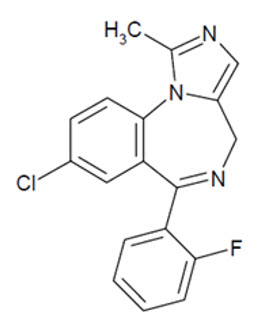
What is Nayzilam (Midazolam)?
Approved To Treat
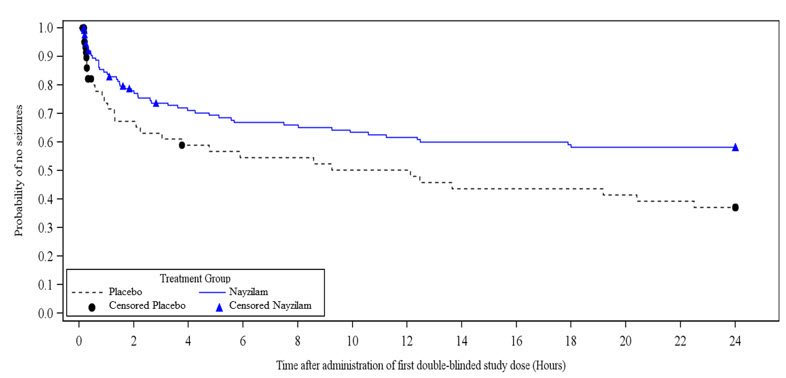
Related Clinical Trials
Summary: The purpose of this study is to assess the effectiveness of oral midazolam on the delivery of care in elderly patients with moderat to severe neurocognitive disorders and opposing care.
Summary: The proposed study is a single-center, phase II, randomized, double-blind, parallel-group, active-placebo-controlled trial of intravenous low-dose ketamine in patients with MS fatigue.
Summary: This is a First In Human (FIH), multicenter, open-label, Phase I/II study to evaluate safety, tolerability, Pharmacokinetics (PK), pharmacodynamics, and efficacy of MT-4561 in patients with advanced solid tumors. This study will be conducted in 3 parts. Part 1 is aimed at evaluating safety, tolerability, PK and pharmacodynamics of MT-4561 and determining the Maximum Tolerated Dose (MTD) using the ...
Related Latest Advances
Brand Information
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death
- The use of benzodiazepines, including NAYZILAM, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing NAYZILAM and throughout treatment, assess each patient's risk for abuse, misuse, and addiction
- The continued use of benzodiazepines may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although NAYZILAM is indicated only for intermittent use
- Known hypersensitivity to midazolam.
- Acute narrow-angle glaucoma
- Risks from Concomitant Use with Opioids
- Abuse, Misuse, and Addiction
- Dependence and Withdrawal Reactions After Use of NAYZILAM More Frequently Than Recommended
- Risks of Cardiorespiratory Adverse Reactions
- CNS Depression from Concomitant Use with Other CNS Depressants or Moderate or Strong CYP3A4 Inhibitors
- Suicidal Behavior and Ideation
- Impaired Cognitive Function
- Glaucoma
- Neonatal Sedation and Withdrawal Syndrome
- Other Adverse Reactions