Brand Name
Tavneos
Generic Name
Avacopan
View Brand Information FDA approval date: October 18, 2021
Classification: Complement 5a Receptor Antagonist
Form: Capsule
What is Tavneos (Avacopan)?
TAVNEOS is indicated as an adjunctive treatment of adult patients with severe active anti-neutrophil cytoplasmic autoantibody -associated vasculitis (granulomatosis with polyangiitis and microscopic polyangiitis ) in combination with standard therapy including glucocorticoids. TAVNEOS does not eliminate glucocorticoid use. TAVNEOS is a complement 5a receptor antagonist indicated as an adjunctive treatment of adult patients with severe active anti-neutrophil cytoplasmic autoantibody -associated vasculitis (granulomatosis with polyangiitis and microscopic polyangiitis ) in combination with standard therapy including glucocorticoids. TAVNEOS does not eliminate glucocorticoid use.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
A Phase 3, Open-label, Uncontrolled Single-arm Study to Evaluate the Efficacy, Pharmacokinetics, and Safety of Avacopan in Combination With a Rituximab or a Cyclophosphamide-containing Regimen in Children From 6 Years to < 18 Years of Age With Active ANCA-associated Vasculitis (AAV)
Summary: The main objective of this study is to explore the efficacy of avacopan in participants affected by AAV.
A Randomized, Double-blind, Placebo-controlled Phase 4 Clinical Trial to Evaluate the Long-term Safety and Efficacy of Avacopan in Participants With Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis
Summary: The primary objective of this study is to evaluate the long-term safety of avacopan in participants with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
A Multi-Center, Phase II, Open Label, Randomized Trial Evaluating the Efficacy and Safety of Complement 5a Receptor Antagonist Avacopan in Crescentic IgA Nephropathy
Summary: The purpose of this study is to evaluate the efficacy and safety of Avacopan together with low-dose glucocorticoid in the treatment of patients with crescentic Imunoglobulin A Nephropathy (IgAN) and high risk of progression.
Related Latest Advances
Brand Information
TAVNEOS (avacopan)
1INDICATIONS AND USAGE
TAVNEOS is indicated as an adjunctive treatment of adult patients with severe active anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. TAVNEOS does not eliminate glucocorticoid use.
2DOSAGE FORMS AND STRENGTHS
Capsules: 10 mg, opaque, yellow and light orange with CCX168 printed in black.
3CONTRAINDICATIONS
TAVNEOS is contraindicated in patients with serious hypersensitivity reaction to avacopan or to any of the excipients
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Hepatotoxicity
- Hypersensitivity Reactions
- Hepatitis B Virus (HBV) Reactivation
- Serious Infections
4.1Clinical Trials Experience
Because the clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The identification of potential adverse drug reactions was based on safety data from the phase 3 clinical trial in which 330 patients with ANCA-associated vasculitis were randomized 1:1 to either TAVNEOS or prednisone
The most frequent serious adverse reactions reported more frequently in patients treated with TAVNEOS than with prednisone were pneumonia (4.8% TAVNEOS vs. 3.7% prednisone), GPA (3.0% TAVNEOS vs. 0.6% prednisone), acute kidney injury (1.8% TAVNEOS vs. 0.6% prednisone), and urinary tract infection (1.8% TAVNEOS vs. 1.2% prednisone). Within 52 weeks, 4 patients in the prednisone treatment group (2.4%) and 2 patients in the TAVNEOS group (1.2%) died. There were no deaths in the phase 2 trials.
In the phase 3 trial, seven patients (4.2%) in the TAVNEOS treatment group and 2 patients (1.2%) in the prednisone treatment group discontinued treatment due to hepatic-related adverse reactions, including hepatobiliary adverse reactions and liver enzymes abnormalities. The most frequent adverse reaction that led to drug discontinuation reported by > 1 patient and more frequently reported in patients treated with TAVNEOS was hepatic function abnormal (1.8%).
The most common adverse reactions that occurred in ≥5% of patients and higher in the TAVNEOS group as compared with the prednisone group are listed in Table 1.
5DESCRIPTION
TAVNEOS (avacopan) capsules contain avacopan, a C5aR antagonist. Avacopan is a chiral molecule containing two stereocenters and has a chemical name of (2

Avacopan is a white to pale yellow crystalline solid that is soluble in organic solvents and practically insoluble in water.
TAVNEOS is available as a 10 mg capsule for oral administration. The capsules include the following inactive ingredients: Polyethylene glycol 4000 (PEG-4000), Polyoxyl-40 hydrogenated castor oil. The capsules are a light orange and yellow opaque bicolor gelatin capsule with a clear gelatin sealing band. The top half of the capsule is printed with "CCX168" in black ink. The capsule shell contains gelatin, red iron oxide, yellow iron oxide, and titanium dioxide, and the capsule sealing band contains gelatin and polysorbate 80.
6CLINICAL STUDIES
The efficacy and safety of TAVNEOS was evaluated in a double-blind, active-controlled, phase 3 clinical trial (NCT02994927) in 330 patients with newly diagnosed or relapsed ANCA-associated vasculitis who were randomized 1:1 to one of the following treatment groups:
- TAVNEOS group (N = 166): Patients received 30 mg avacopan twice daily for 52 weeks plus prednisone-matching placebo for 20 weeks
- Prednisone group (N = 164): Patients received avacopan-matched placebo twice daily for 52 weeks plus prednisone (tapered from 60 mg/day to 0 over 20 weeks)
All patients in both groups received one of the following standard immunosuppressive regimens:
- IV cyclophosphamide 15 mg/kg IV up to 1.2 g maximum every 2 to 3 weeks for 13 weeks followed by oral azathioprine 1 mg/kg/day with titration up to 2 mg/kg/day (or mycophenolate mofetil at a target dose of 2 g/day if azathioprine was contraindicated) from Week 15 onwards
- Oral cyclophosphamide 2 mg/kg/day (maximum 200 mg/day) for 14 weeks followed by azathioprine 1 mg/kg/day with titration up to 2 mg/kg/day (or mycophenolate mofetil at a target dose of 2 g/day if azathioprine was contraindicated) from Week 15 onwards
- IV rituximab 375 mg/m
Glucocorticoids were allowed as pre-medication for rituximab to reduce hypersensitivity reactions, taper after glucocorticoids given during the Screening period, treatment of persistent vasculitis, worsening of vasculitis, or relapses, as well as for non-vasculitis reasons such as adrenal insufficiency.
Randomization was stratified based on 3 factors: newly-diagnosed or relapsing ANCA-associated vasculitis, proteinase 3 positive or myeloperoxidase positive ANCA-associated vasculitis, and standard immunosuppressive regimen. The primary endpoints of the study were disease remission at Week 26 and sustained disease remission at Week 52. Disease remission was defined as achieving a Birmingham Vasculitis Activity Score (BVAS) of 0 and no use of glucocorticoids for treatment of ANCA-associated vasculitis from Week 22 to Week 26. Sustained remission was defined as remission at Week 26 and remission at Week 52, without relapse between Week 26 and Week 52. Remission at Week 52 was defined as BVAS of 0 and no use of glucocorticoids for treatment of ANCA-associated vasculitis from Week 48 to Week 52. Relapse was defined as occurrence of one major item, at least 3 non-major items, or 1 or 2 non-major items for at least 2 consecutive visits on the BVAS after remission (BVAS of 0) had been achieved.
The two treatment groups were well balanced regarding baseline demographics and disease characteristics of patients in this trial. The mean patient age was 60.9 years. Most patients were male (56.4%), Caucasian (84.2%), and had newly diagnosed disease (69.4%). Patients had either GPA (54.8%) or MPA (45.2%) and had presence of anti-PR3 (43.0%) or anti-MPO (57.0%) antibodies. Mean baseline BVAS was 16.2; patients most commonly had manifestations within the renal component (81.2%), general component (68.2%), ear/nose/throat component (43.6%), and chest component (43.0%). Approximately 65% of patients received rituximab, 31% received IV cyclophosphamide, and 4% received oral cyclophosphamide.
7HOW SUPPLIED/STORAGE AND HANDLING
TAVNEOS (avacopan) capsule is supplied as a 10 mg, hard, opaque yellow and light orange capsule with "CCX168" printed in black.
- Bottle containing 180 capsules with child resistant induction seal closure (NDC 73556-168-01)
- Bottle containing 30 capsules with child resistant induction seal closure (NDC 73556-168-02)
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
- Dosage and Administration: Instruct the patient that TAVNEOS should be swallowed whole. TAVNEOS should not be chewed or crushed. If a dose is missed, instruct the patient to take the next scheduled dose
- Hypersensitivity Reactions: Advise patients to seek immediate medical attention when experiencing any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, and difficulty in swallowing or breathing) and to discontinue the drug until they have consulted with the prescribing physician
- Hepatotoxicity: Inform patients of the signs and symptoms of hepatic adverse reactions. Advise patients to contact their healthcare provider immediately for signs or symptoms of liver problems; yellowing of the skin or the white part of the eyes (jaundice), dark or brown (tea colored) urine, pain on the upper right side of the stomach area (abdomen), bleeding or bruising
- Infections: Inform patients that serious infections have been reported in patients receiving TAVNEOS, including reactivation of hepatitis B infection. Instruct patients to contact their healthcare provider immediately if they develop any signs or symptoms of an infection
- Lactation: Consider benefits/risk during lactation
9PRINCIPAL DISPLAY PANEL - 180 Capsule Bottle Carton
NDC 73556-168-01
TAVNEOS
10 mg
PHARMACIST: Dispense with accompanying
180 capsules
AMGEN

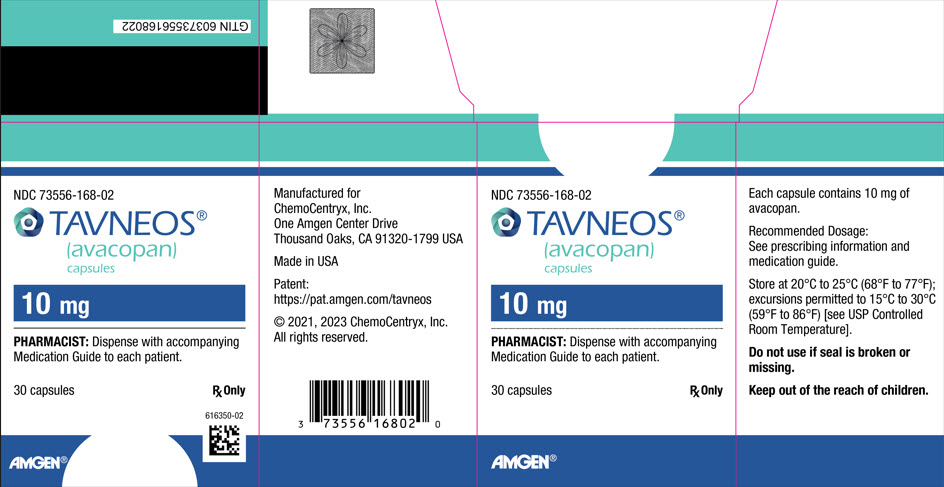
10PRINCIPAL DISPLAY PANEL - 30 Capsule Bottle Carton
NDC 73556-168-02
TAVNEOS
10 mg
PHARMACIST: Dispense with accompanying
30 capsules
AMGEN