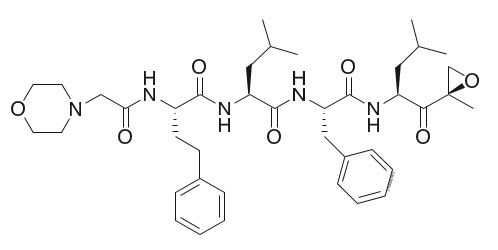
Kyprolis
What is Kyprolis (Carfilzomib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to learn about the study medicine called elranatamab.This study aims to compare elranatamab to other medicines for the treatment of MM (a type of cancer). This study is seeking participants who: * Are 18 years of age or older and have MM. * Have received treatments before for MM. * Have MM that has returned or not responded to their most recent treatment. Half of the p...
Summary: The purpose of this study is to provide ongoing access to study treatments for participants with multiple myeloma or smoldering multiple myeloma benefiting from treatment in certain Janssen Research and Development (R\&D) studies that use daratumumab as part of the study treatment regimen: access for all participants regardless of treatment group in daratumumab studies and access to participants i...
Summary: The main purpose of this study is to measure the efficacy (Myeloma response) of subcutaneous (SC) isatuximab treatment in combination with carfilzomib and dexamethasone in adult participants with RRMM having received 1 to 3 prior lines of therapy, and to characterize the PK of isatuximab in combination with carfilzomib and dexamethasone after manual and On Body Delivery System (OBDS) administratio...
Related Latest Advances
Brand Information
- Cardiac Toxicities
- Acute Renal Failure
- Tumor Lysis Syndrome
- Pulmonary Toxicity
- Pulmonary Hypertension
- Dyspnea
- Hypertension
- Venous Thrombosis
- Infusion-Related Reactions
- Hemorrhage
- Thrombocytopenia
- Hepatic Toxicity and Hepatic Failure
- Thrombotic Microangiopathy
- Posterior Reversible Encephalopathy Syndrome
- Progressive Multifocal Leukoencephalopathy