Generic Name
Fingolimod
Brand Names
Gilenya, Tascenso Odt
FDA approval date: September 21, 2010
Classification: Sphingosine 1-phosphate Receptor Modulator
Form: Tablet, Capsule
What is Gilenya (Fingolimod)?
Fingolimod capsules are indicated for the treatment of relapsing forms of multiple sclerosis , to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 years of age and older. Fingolimod is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of multiple sclerosis , to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 years of age and older.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Gilenya (Fingolimod hcl)
1INDICATIONS AND USAGE
GILENYA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in patients 10 years of age and older.
2DOSAGE FORMS AND STRENGTHS
GILENYA is available as:
- 0.25 mg hard capsules with an ivory opaque body and cap, with black radial imprint “FTY 0.25 mg” on the cap and a black radial band on the capsule body.
- 0.5 mg hard capsules with a white opaque body and bright yellow cap imprinted with “FTY 0.5 mg” on the cap and 2 radial bands imprinted on the capsule body with yellow ink.
3CONTRAINDICATIONS
GILENYA is contraindicated in patients who have:
- in the last 6 months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization or Class III/IV heart failure
- a history or presence of Mobitz Type II second-degree or third-degree AV block or sick sinus syndrome, unless patient has a functioning pacemaker
- a baseline QTc interval ≥ 500 msec
- cardiac arrhythmias requiring anti-arrhythmic treatment with Class Ia or Class III anti-arrhythmic drugs
- had a hypersensitivity reaction to fingolimod or any of the excipients in GILENYA. Observed reactions include rash, urticaria and angioedema upon treatment initiation
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Bradyarrhythmia and Atrioventricular Blocks
- Infections
- Progressive Multifocal Leukoencephalopathy
- Macular Edema
- Liver Injury
- Posterior Reversible Encephalopathy Syndrome
- Respiratory Effects
- Fetal Risk
- Severe Increase in Disability After Stopping GILENYA
- Tumefactive Multiple Sclerosis
- Increased Blood Pressure
- Malignancies
- Immune System Effects Following GILENYA Discontinuation
- Hypersensitivity Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
In clinical trials (Studies 1, 2, and 3), a total of 1212 patients with relapsing forms of multiple sclerosis received GILENYA 0.5 mg. This included 783 patients who received GILENYA 0.5 mg in the 2-year placebo-controlled trials (Studies 1 and 3) and 429 patients who received GILENYA 0.5 mg in the 1-year active-controlled trial (Study 2). The overall exposure in the controlled trials was equivalent to 1716 person-years. Approximately 1000 patients received at least 2 years of treatment with GILENYA 0.5 mg. In all clinical studies, including uncontrolled extension studies, the exposure to GILENYA 0.5 mg was approximately 4119 person-years.
In placebo-controlled trials, the most frequent adverse reactions (incidence ≥ 10% and greater than placebo) for GILENYA 0.5 mg were headache, liver transaminase elevation, diarrhea, cough, influenza, sinusitis, back pain, abdominal pain, and pain in extremity. Adverse events that led to treatment discontinuation and occurred in more than 1% of patients taking GILENYA 0.5 mg, were serum transaminase elevations (4.7% compared to 1% on placebo) and basal cell carcinoma (1% compared to 0.5% on placebo).
Table 1 lists adverse reactions in clinical studies in adults that occurred in ≥ 1% of GILENYA-treated patients and ≥ 1% higher rate than for placebo.
Adverse reactions of seizure, dizziness, pneumonia, eczema, and pruritus were also reported in Studies 1 and 3, but did not meet the reporting rate criteria for inclusion in Table 1 (difference was less than 1%).
Adverse reactions with GILENYA 0.5 mg in Study 2, the 1-year active-controlled (versus interferon beta-1a) study were generally similar to those in Studies 1 and 3.
Vascular Events
Vascular events, including ischemic and hemorrhagic strokes, and peripheral arterial occlusive disease were reported in premarketing clinical trials in patients who received GILENYA doses (1.25 mg to 5 mg) higher than recommended for use in MS. Similar events have been reported with GILENYA in the postmarketing setting although a causal relationship has not been established.
Seizure
Cases of seizures, including status epilepticus, have been reported with the use of GILENYA in clinical trials and in the postmarketing setting in adults
Pediatric Patients 10 Years of Age and Older
In the controlled pediatric trial (Study 4), the safety profile in pediatric patients receiving GILENYA 0.25 mg or 0.5 mg daily was similar to that seen in adult patients.
In the pediatric study, cases of seizures were reported in 5.6% of GILENYA-treated patients and 0.9% of interferon beta-1a-treated patients
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of GILENYA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Hemolytic anemia and thrombocytopenia
Hepatobiliary Disorders: Liver injury [see Warnings and Precautions (5.5)]
Infections: Infections, including cryptococcal infections [see Warnings and Precautions (5.2)], human papilloma virus (HPV) infection, including papilloma, dysplasia, warts and HPV-related cancer [see Warnings and Precautions (5.2)], progressive multifocal leukoencephalopathy [see Warnings and Precautions (5.3)]
Musculoskeletal and Connective Tissue Disorders: Arthralgia, myalgia
Nervous System Disorders: Posterior reversible encephalopathy syndrome [see Warnings and Precautions (5.6)], seizures, including status epilepticus [see Adverse Reactions (6.1)]
Neoplasms, Benign, Malignant, and Unspecified (including cysts and polyps): Melanoma, Merkel cell carcinoma, cutaneous T-cell lymphoma (including mycosis fungoides), Kaposi’s sarcoma, squamous cell carcinoma [see Warnings and Precautions (5.12)]
Skin and Subcutaneous Tissue Disorders: Hypersensitivity [see Warnings and Precautions (5.14)]
5OVERDOSAGE
GILENYA can induce bradycardia as well as AV conduction blocks (including complete AV block). The decline in heart rate usually starts within 1 hour of the first dose and is maximal within 6 hours in most patients
Neither dialysis nor plasma exchange results in removal of fingolimod from the body.
6DESCRIPTION
Fingolimod is a sphingosine 1-phosphate receptor modulator.
Chemically, fingolimod is 2-amino-2-[2-(4-octylphenyl)ethyl]propan-1,3-diol hydrochloride. Its structure is shown below:
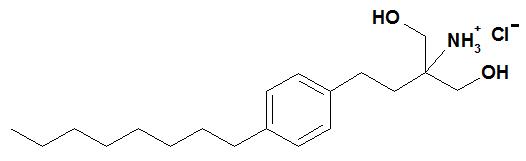
Fingolimod hydrochloride is a white to practically white powder that is freely soluble in water and alcohol and soluble in propylene glycol. It has a molecular weight of 343.93 g/mol.
GILENYA is provided as 0.25 mg and 0.5 mg hard gelatin capsules for oral use.
Each 0.25 mg capsule contains 0.28 mg of fingolimod hydrochloride, equivalent to 0.25 mg fingolimod.
Each 0.5 mg capsule contains 0.56 mg of fingolimod hydrochloride, equivalent to 0.5 mg of fingolimod.
Each GILENYA 0.25 mg capsule contains the following inactive ingredients: gelatin, hydroxypropylbetadex, hydroxypropylcellulose, magnesium stearate, mannitol, titanium dioxide, and yellow iron oxide.
Each GILENYA 0.5 mg capsule contains the following inactive ingredients: gelatin, magnesium stearate, mannitol, titanium dioxide, and yellow iron oxide.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Tell patients not to discontinue GILENYA without first discussing this with the prescribing healthcare provider. Advise patients to contact their healthcare provider if they accidently take more GILENYA than prescribed.
Cardiac Effects
Advise patients that initiation of GILENYA treatment results in a transient decrease in heart rate. Inform patients that they will need to be observed in the healthcare provider’s office or other facility for at least 6 hours after the first dose, after reinitiation if treatment is interrupted or discontinued for certain periods, and after the dosage is increased
Risk of Infections
Inform patients that they may have an increased risk of infections, some of which could be life-threatening, when taking GILENYA, and that they should contact their healthcare provider if they develop symptoms of infection. Advise patients that the use of some vaccines should be avoided during treatment with GILENYA and for 2 months after discontinuation. Recommend to patients that they delay treatment with GILENYA until after VZV vaccination if they have not had chickenpox or a previous VZV vaccination. Inform patients that prior or concomitant use of drugs that suppress the immune system may increase the risk of infection
Progressive Multifocal Leukoencephalopathy
Inform patients that cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients who received GILENYA. Inform the patient that PML is characterized by a progression of deficits and usually leads to death or severe disability over weeks or months. Instruct the patient of the importance of contacting their healthcare provider if they develop any symptoms suggestive of PML. Inform the patient that typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes
Macular Edema
Advise patients that GILENYA may cause macular edema, and that they should obtain an eye exam near the start of treatment with GILENYA, have their eyes monitored periodically by an eye care professional while receiving therapy, and contact their healthcare provider if they experience any changes in their vision while taking GILENYA. Inform patients with diabetes mellitus or a history of uveitis that their risk of macular edema is increased
Hepatic Effects
Inform patients that GILENYA may cause liver injury. Advise patients that they should contact their healthcare provider if they have any unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine
Posterior Reversible Encephalopathy Syndrome
Advise patients to immediately report to their healthcare provider any symptoms involving sudden onset of severe headache, altered mental status, visual disturbances, or seizure. Inform patients that delayed treatment could lead to permanent neurological sequelae
Respiratory Effects
Advise patients that they should contact their healthcare provider if they experience new onset or worsening of dyspnea
Fetal Risk
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy
- Advise female patients of reproductive potential to use effective contraception during treatment with GILENYA and for 2 months after the final dose
Severe Increase in Disability After Stopping GILENYA
Inform patients that severe increase in disability has been reported after discontinuation of GILENYA. Advise patients to contact their healthcare provider if they develop worsening symptoms of MS following discontinuation of GILENYA
Malignancies
Advise patients that the risk of basal cell carcinoma and melanoma is increased with use of GILENYA and that cases of squamous cell carcinoma, Merkel cell carcinoma, and Kaposi’s sarcoma have been reported. Advise patients that any suspicious skin lesions should be promptly evaluated. Advise patients to limit exposure to sunlight and ultraviolet light by wearing protective clothing and using a sunscreen with a high protection factor. Inform patients that lymphoma has also occurred in patients receiving GILENYA
Persistence of GILENYA Effects After Drug Discontinuation
Advise patients that GILENYA remains in the blood and continues to have effects, including decreased blood lymphocyte counts, for up to 2 months following the last dose
Hypersensitivity Reactions
Advise patients that GILENYA may cause hypersensitivity reactions, including rash, urticaria, and angioedema. Advise patients to contact their healthcare provider if they have any symptoms associated with hypersensitivity
Pregnancy
Instruct patients that if they are pregnant or plan to become pregnant while taking GILENYA they should inform their healthcare provider
GILENYA is a registered trademark of Novartis, AG.
Distributed by:
© Novartis
T2025-53
8PRINCIPAL DISPLAY PANEL
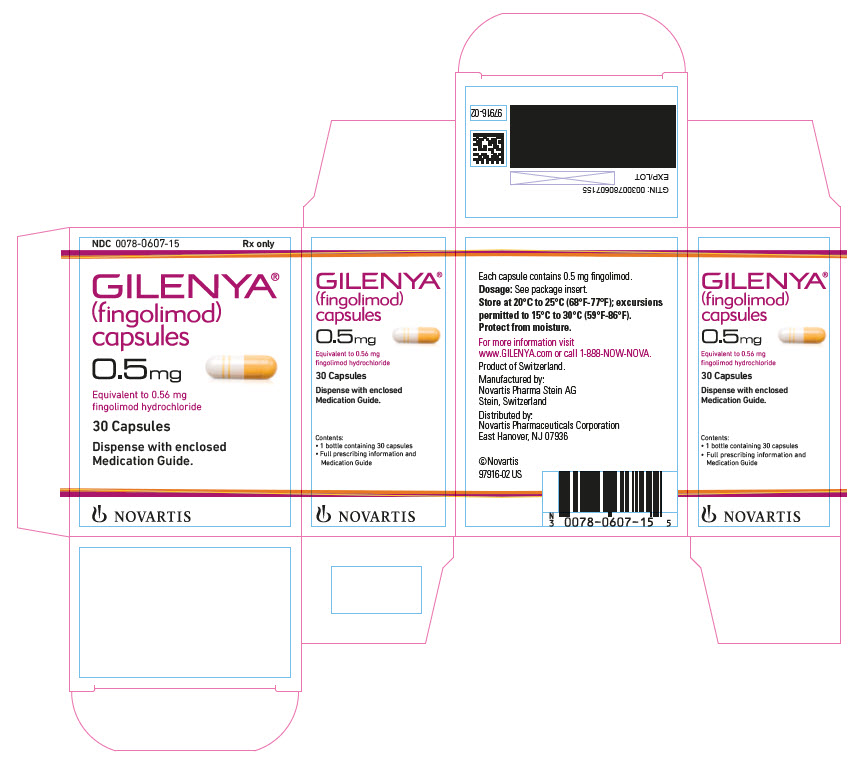
NDC 0078-0607-15 Rx only
GILENYA
(fingolimod)
capsules
0.5 mg
Equivalent to 0.56 mg
30 Capsules
Dispense with enclosed Medication Guide.
NOVARTIS
9PRINCIPAL DISPLAY PANEL
NDC 0078-0965-89
GILENYA
(fingolimod)
capsules
0.25 mg
Equivalent to 0.28 mg
7 Capsules
Rx only
This package includes a 1-week supply of capsules.
Dispense with enclosed Medication Guide.
NOVARTIS
