Revlimid
What is Revlimid (Lenalidomide)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: CO43923 is a platform study that will evaluate the safety, efficacy, and pharmacokinetics (PK) of multiple treatment combinations, as monotherapy or in combination, in participants with multiple myeloma (MM). The study is designed with the flexibility to open new treatment substudies as new treatments become available. Information regarding the opened substudies are found below.
Summary: This phase I trial tests the safety, side effects, best dose and effectiveness of lenalidomide when added to nivolumab and the usual drugs (rituximab and methotrexate) in patients with primary central nervous system (CNS) lymphoma. Lenalidomide may stop or slow primary CNS lymphoma by blocking the growth of new blood vessels necessary for tumor growth. Immunotherapy with monoclonal antibodies, suc...
Summary: This phase II trial tests how well venetoclax, ibrutinib, prednisone, obinutuzumab, and Revlimid® (ViPOR) works in treating patients with CD10 negative diffuse large B-cell lymphoma (DLBCL) and high-grade lymphoma with MYC and BCL2 rearrangements that has come back after a period of improvement (relapsed) and/or that has not responded to previous treatment (refractory). Venetoclax is in a class of...
Related Latest Advances
Brand Information
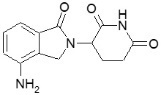
- 2.5 mg, white and blue-green opaque hard capsules imprinted "REV" on one half and "2.5 mg" on the other half in black ink
- 5 mg, white opaque capsules imprinted "REV" on one half and "5 mg" on the other half in black ink
- 10 mg, blue/green and pale yellow opaque capsules imprinted "REV" on one half and "10 mg" on the other half in black ink
- 15 mg, powder blue and white opaque capsules imprinted "REV" on one half and "15 mg" on the other half in black ink
- 20 mg, powder blue and blue-green opaque hard capsules imprinted "REV" on one half and "20 mg" on the other half in black ink
- 25 mg, white opaque capsules imprinted "REV" on one half and "25 mg" on the other half in black ink
- Embryo-Fetal Toxicity
- Hematologic Toxicity
- Venous and Arterial Thromboembolism
- Increased Mortality in Patients with CLL
- Second Primary Malignancies
- Increased Mortality in Patients with MM When Pembrolizumab Is Added to a Thalidomide Analogue and Dexamethasone
- Hepatotoxicity
- Severe Cutaneous Reactions
- Tumor Lysis Syndrome
- Tumor Flare Reactions
- Impaired Stem Cell Mobilization
- Thyroid Disorders
- Early Mortality in Patients with MCL
- Hypersensitivity