Dupixent
What is Dupixent (Dupilumab)?
Approved To Treat
Related Clinical Trials
Summary: This study is researching an experimental drug called dupilumab (called study drug). The study is focused on children with active eosinophilic esophagitis (EoE; an inflammatory disease of the esophagus) which impacts feeding and nourishment. The aim of the study is to see how safe, tolerable, and effective the study drug is when given for 24 weeks to children with active EoE. The study is looking ...
Summary: This is a parallel, Phase 3, 2-arm study to evaluate the efficacy and long-term safety of dupilumab treatment in children 2 to \<6 years of age with uncontrolled asthma and/or recurrent severe asthmatic wheeze. The study will be conducted in 2 parts. Part A will be a 52-week, randomized, double-blind, placebo-controlled study to assess the safety and efficacy of dupilumab in children aged 2 to \<6...
Summary: The purpose of this study is to evaluate how well JNJ-95475939 works as compared to placebo in participants with moderate to severe atopic dermatitis.
Related Latest Advances
Brand Information
- Injection: 300 mg/2 mL (150 mg/mL)
- Injection: 200 mg/1.14 mL (175 mg/mL)
- Injection: 300 mg/2 mL (150 mg/mL)
- Injection: 200 mg/1.14 mL (175 mg/mL)
- Hypersensitivity
- Conjunctivitis and Keratitis
- Psoriasis
- Arthralgia and Psoriatic Arthritis
- Parasitic (Helminth) Infections
- Immune system disorders: angioedema
- Musculoskeletal system disorders: psoriatic arthritis
- Skin and subcutaneous tissue disorders: Facial skin reactions, including erythema, rash, scaling, edema, papules, pruritus, burning, and pain; new-onset psoriasis, vasculitis
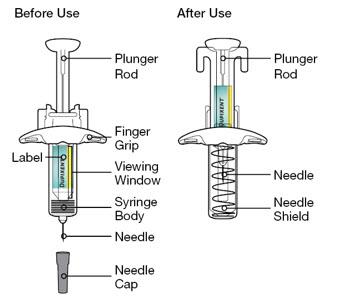
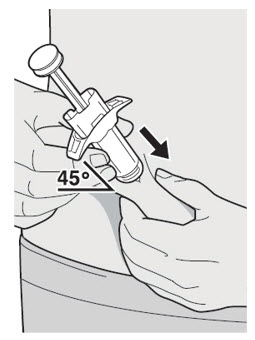
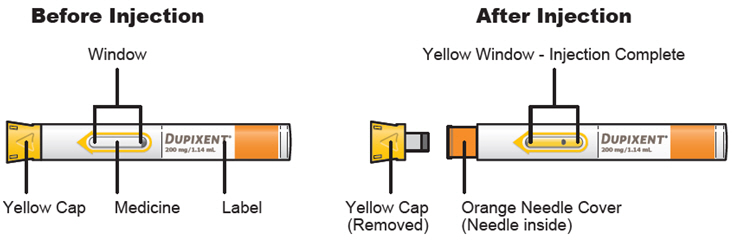
 Do not pull off the Needle Cap until you are ready to inject.
Do not pull off the Needle Cap until you are ready to inject. Do not use the DUPIXENT Syringe if it has been dropped on a hard surface or damaged.
Do not use the DUPIXENT Syringe if it has been dropped on a hard surface or damaged.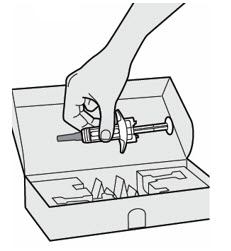
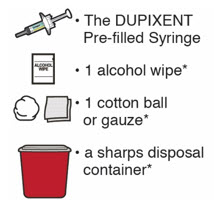
- the DUPIXENT Pre-filled Syringe
- 1 alcohol wipe*
- 1 cotton ball or gauze*
- a sharps disposal container* (See
- you have the correct medicine and dose.
- the expiration date on the Single-Dose Pre-filled Syringe has not passed.
 Do not use the DUPIXENT Syringe if the expiration date has passed.
Do not use the DUPIXENT Syringe if the expiration date has passed.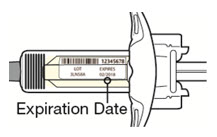
 Do not use the DUPIXENT Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.
Do not use the DUPIXENT Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.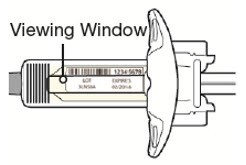
 Do not heat the DUPIXENT Syringe.
Do not heat the DUPIXENT Syringe. Do not put the DUPIXENT Syringe into direct sunlight.
Do not put the DUPIXENT Syringe into direct sunlight. Do not keep DUPIXENT Syringes at room temperature for more than 14 days. Throw away (dispose of) any DUPIXENT Syringes that have been left at room temperature for longer than 14 days.
Do not keep DUPIXENT Syringes at room temperature for more than 14 days. Throw away (dispose of) any DUPIXENT Syringes that have been left at room temperature for longer than 14 days.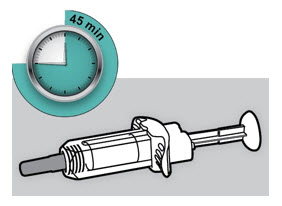
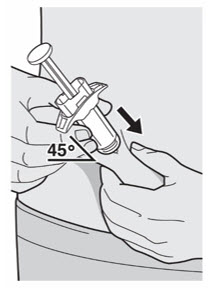
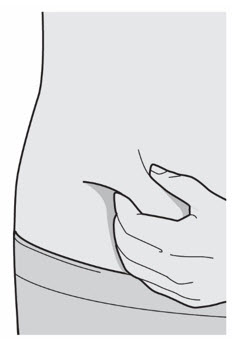
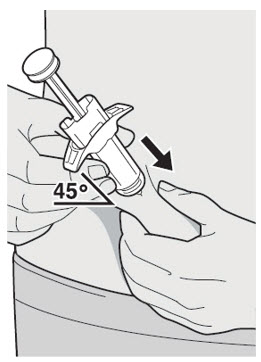
- You can inject into your thigh or stomach, except for the 2 inches (5 cm) around your belly button (navel).
- If a caregiver injects your dose, they can also use the outer area of the upper arm.
- Choose a different site each time you inject DUPIXENT.
 Do not inject into skin that is tender, damaged, bruised or scarred.
Do not inject into skin that is tender, damaged, bruised or scarred. 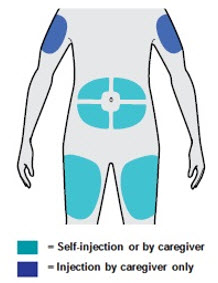
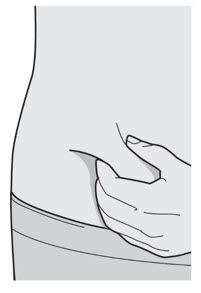
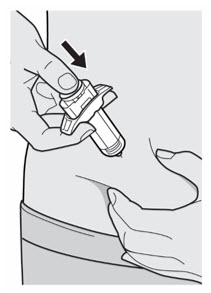
 Do not touch the injection site again or blow on it before the injection.
Do not touch the injection site again or blow on it before the injection.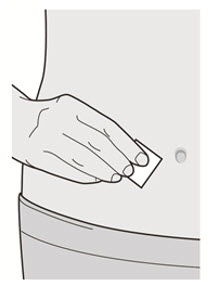
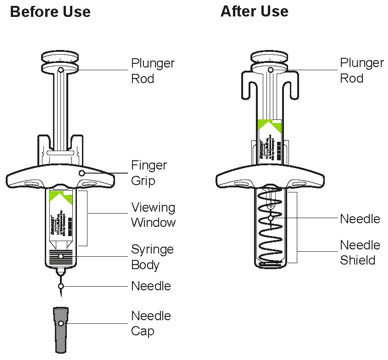
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on. Do not touch the Needle.
Do not touch the Needle.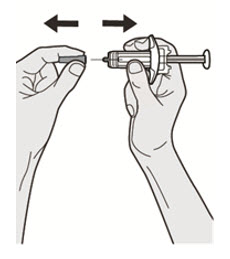
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on. Do not rub your skin after the injection.
Do not rub your skin after the injection.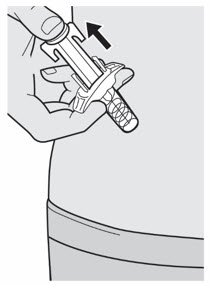
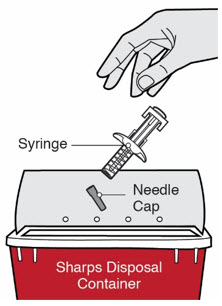
 Do not dispose of (throw away) Needles, DUPIXENT Syringes, and Needle Caps in your household trash.
Do not dispose of (throw away) Needles, DUPIXENT Syringes, and Needle Caps in your household trash.- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on.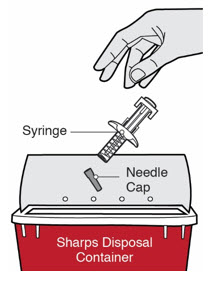

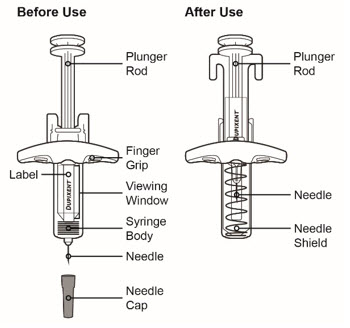
 Do not pull off the Needle Cap until you are ready to inject.
Do not pull off the Needle Cap until you are ready to inject. Do not use the DUPIXENT Syringe if it has been dropped on a hard surface or damaged.
Do not use the DUPIXENT Syringe if it has been dropped on a hard surface or damaged.
- the DUPIXENT Pre-filled Syringe
- 1 alcohol wipe*
- 1 cotton ball or gauze*
- a sharps disposal container* (See

- you have the correct medicine and dose.
- the expiration date on the Single-Dose Pre-filled Syringe has not passed.
 Do not use the DUPIXENT Syringe if the expiration date has passed.
Do not use the DUPIXENT Syringe if the expiration date has passed.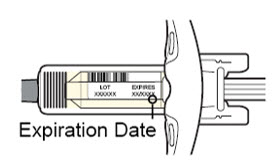
 Do not use the DUPIXENT Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.
Do not use the DUPIXENT Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.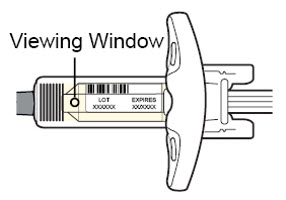
 Do not heat the DUPIXENT Syringe.
Do not heat the DUPIXENT Syringe. Do not put the DUPIXENT Syringe into direct sunlight.
Do not put the DUPIXENT Syringe into direct sunlight. Do not keep DUPIXENT Syringes at room temperature for more than 14 days. Throw away (dispose of) any DUPIXENT Syringes that have been left at room temperature for longer than 14 days.
Do not keep DUPIXENT Syringes at room temperature for more than 14 days. Throw away (dispose of) any DUPIXENT Syringes that have been left at room temperature for longer than 14 days.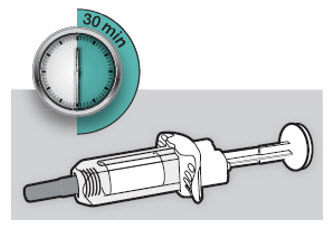
- You can inject into your thigh or stomach, except for the 2 inches (5 cm) around your belly button (navel).
- If a caregiver injects your dose, they can also use the outer area of the upper arm.
- Choose a different site each time you inject DUPIXENT.
 Do not inject into skin that is tender, damaged, bruised or scarred.
Do not inject into skin that is tender, damaged, bruised or scarred. 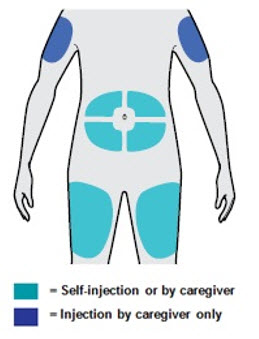
 Do not touch the injection site again or blow on it before the injection.
Do not touch the injection site again or blow on it before the injection.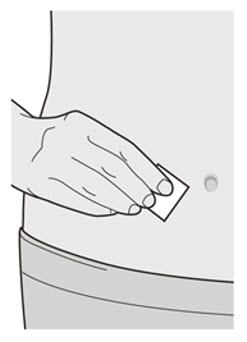
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on. Do not touch the Needle.
Do not touch the Needle.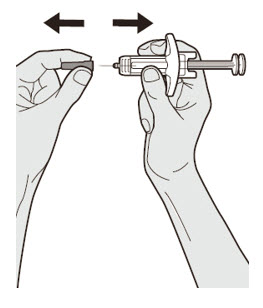
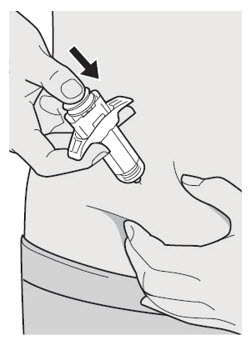
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on. Do not rub your skin after the injection.
Do not rub your skin after the injection.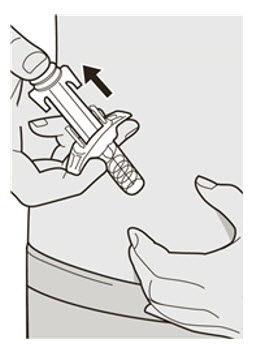
 Do not dispose of (throw away) Needles, DUPIXENT Syringes, and Needle Caps in your household trash.
Do not dispose of (throw away) Needles, DUPIXENT Syringes, and Needle Caps in your household trash.- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on.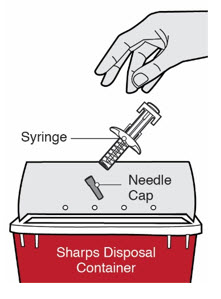

 Do not pull off the Needle Cap until you are ready to inject.
Do not pull off the Needle Cap until you are ready to inject. Do not use the DUPIXENT Syringe if it has been dropped on a hard surface or damaged.
Do not use the DUPIXENT Syringe if it has been dropped on a hard surface or damaged.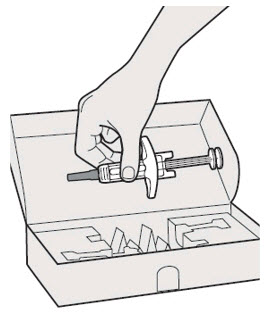
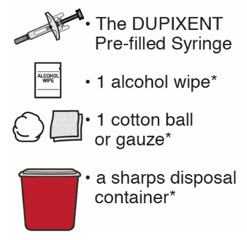
- the DUPIXENT Pre-filled Syringe
- 1 alcohol wipe*
- 1 cotton ball or gauze*
- a sharps disposal container* (See
- you have the correct medicine and dose.
- the expiration date on the Single-Dose Pre-filled Syringe has not passed.
 Do not use the DUPIXENT Syringe if the expiration date has passed.
Do not use the DUPIXENT Syringe if the expiration date has passed.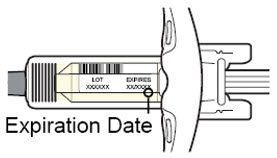
 Do not use the DUPIXENT Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.
Do not use the DUPIXENT Syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.
 Do not heat the DUPIXENT Syringe.
Do not heat the DUPIXENT Syringe. Do not put the DUPIXENT Syringe into direct sunlight.
Do not put the DUPIXENT Syringe into direct sunlight. Do not keep DUPIXENT Syringes at room temperature for more than 14 days. Throw away (dispose of) any DUPIXENT Syringes that have been left at room temperature for longer than 14 days.
Do not keep DUPIXENT Syringes at room temperature for more than 14 days. Throw away (dispose of) any DUPIXENT Syringes that have been left at room temperature for longer than 14 days.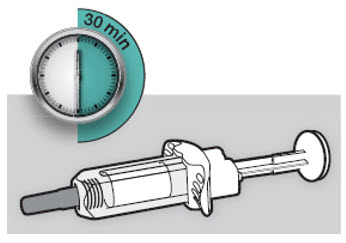
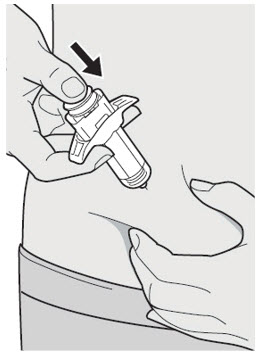
- You can inject into the thigh, outer area of the upper arm or stomach, except for the 2 inches (5 cm) around the belly button (navel).
- Choose a different site each time you inject DUPIXENT.
 Do not inject into skin that is tender, damaged, bruised or scarred.
Do not inject into skin that is tender, damaged, bruised or scarred. 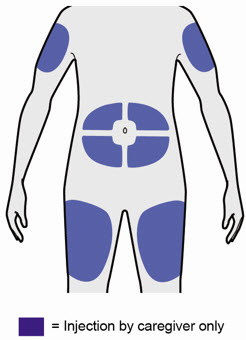
 Do not touch the injection site again or blow on it before the injection.
Do not touch the injection site again or blow on it before the injection.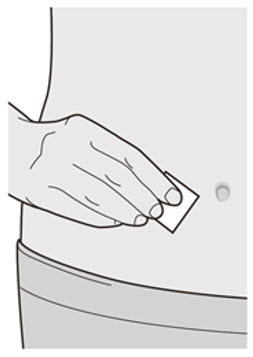
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on. Do not touch the Needle.
Do not touch the Needle.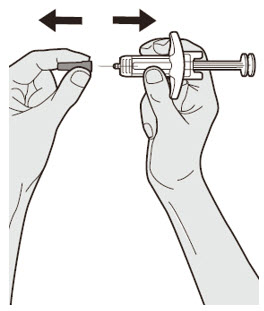
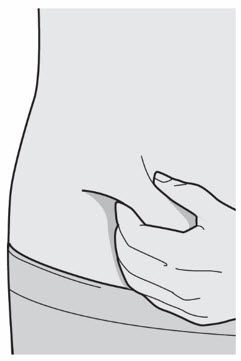
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on. Do not rub the skin after the injection.
Do not rub the skin after the injection.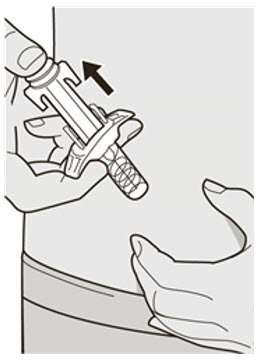
 Do not dispose of (throw away) Needles, DUPIXENT Syringes, and Needle Caps in your household trash.
Do not dispose of (throw away) Needles, DUPIXENT Syringes, and Needle Caps in your household trash.- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container
 Do not put the Needle Cap back on.
Do not put the Needle Cap back on.