Brand Name
Arcalyst
Generic Name
Rilonacept
View Brand Information FDA approval date: February 27, 2008
Form: Injection
What is Arcalyst (Rilonacept)?
ARCALYST is an interleukin-1 blocker indicated for: Treatment of Cryopyrin-Associated Periodic Syndromes , including Familial Cold Autoinflammatory Syndrome , and Muckle-Wells Syndrome in adults and children 12 years and older.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
A RandomizEd PhAse II TrIal of Rilonacept in Subjects With Cardiac Sarcoidosis (REPAIR-CS)
Summary: The primary objective of this study is to evaluate the effect of rilonacept, added to standard therapy and compared with standard therapy alone, on improvement in myocardial inflammation in subjects with cardiac sarcoidosis after 24 weeks of therapy.
Related Latest Advances
Brand Information
Arcalyst (rilonacept)
1DOSAGE FORMS AND STRENGTHS
For injection: 220 mg of rilonacept as a white to off-white lyophilized powder for reconstitution in single-dose vials.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling.
- Serious Infections
- Risk of Malignancy
- Hypersensitivity Reactions
- Lipid Profile Changes
3.1Clinical Trial Experience
Clinical trials are conducted under widely varying conditions and, as such, adverse reaction rates observed cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described herein reflect exposure to ARCALYST in over 2,000 patients who received at least one dose, including approximately 1700 exposed to 160 mg or more, of whom 151 patients were exposed for at least 6 months and 111 patients for at least one year. These included patients with CAPS and RP, patients with other diseases, and healthy volunteers.
CAPS
Approximately 60 patients with CAPS were treated weekly with 160 mg of ARCALYST. The pivotal trial population included 47 patients with CAPS. These patients were between the ages of 22 and 78 years (average 51 years). Thirty-one patients were female and 16 were male. All of the patients were White/Caucasian. Six pediatric patients (12 to17 years) were enrolled directly into the open-label extension phase of the trial.
Part A of the clinical trial was conducted in patients with CAPS who were naïve to treatment with ARCALYST. Part A of the study was a randomized, double-blind, placebo-controlled, six-week study comparing ARCALYST to placebo
DIRA
In a 2-year, open-label study, 6 pediatric patients with DIRA, 3 years to 6 years of age, received a 2.2 to 4.4 mg/kg dose of ARCALYST once weekly
RP
In the RP phase 3 study, a total of 86 patients received at least one dose of ARCALYST with a median treatment duration of 9 months
Adverse Reactions of Special Interest
Injection-Site Reactions
In patients with CAPS or RP, the most common and consistently reported adverse event associated with ARCALYST was injection-site reaction (ISR). The ISRs included erythema, swelling, pruritus, mass, bruising, inflammation, pain, edema, dermatitis, discomfort, urticaria, vesicles, warmth and hemorrhage. Most injection-site reactions lasted for one to two days.
Infections
During Part A in the CAPS study, the incidence of patients reporting infections was greater with ARCALYST (48%) than with placebo (17%). In Part B, randomized withdrawal, the incidence of infections was similar in the ARCALYST (18%) and the placebo patients (22%). Part A of the trial was initiated in the winter months, while Part B was predominantly performed in the summer months.
In placebo-controlled studies across a variety of patient populations encompassing 360 patients treated with rilonacept and 179 treated with placebo, the incidence of infections was 34% and 27% (2.15 per patient-exposure year and 1.81 per patient-exposure year), respectively, for rilonacept and placebo.
Serious Infections
Six serious infections were reported by four patients during the CAPS clinical program:
One patient receiving ARCALYST for an unapproved indication in another study developed an infection in his olecranon bursa with
Changes in Hematologic Parameters Laboratory Changes
One patient in a study in an unapproved indication developed transient neutropenia (ANC < 1 × 10
Lipid Profile Changes
Patients with CAPS treated with ARCALYST experienced increases in their mean total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides. The mean increases from baseline for total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were 19 mg/dL, 2 mg/dL, 10 mg/dL, and 57 mg/dL, respectively, after 6 weeks of open-label therapy.
3.2Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. The data reflect the percentage of patients whose test results were positive for antibodies to the rilonacept receptor domains in specific assays. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay is highly dependent on several factors including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to rilonacept with the incidence of antibodies in other studies or to other products may be misleading.
Antibodies directed against the receptor domains of rilonacept were detected by an ELISA assay in patients with CAPS after treatment with ARCALYST. Nineteen of 55 patients (35%) who had received ARCALYST for at least 6 weeks tested positive for treatment-emergent binding antibodies on at least one occasion. Of the 19, seven tested positive at the last assessment (Week 18 or 24 of the open-label extension period), and five patients tested positive for neutralizing antibodies on at least one occasion. There was no correlation of antibody activity and either clinical effectiveness or safety.
In the Phase 3 study of patients with RP, there were no patients who tested positive for antibodies at baseline. At any point in time, 26 out of 86 (30%) subjects tested positive at any assessment and of these, 6 tested positive for neutralizing antibodies (NAb). At the last assessment, 10 subjects remained positive for anti-drug antibodies (ADA) and 1 subject remained positive for NAb. There was no correlation of antibody activity and either clinical effectiveness or safety.
4OVERDOSAGE
There have been no reports of overdose with ARCALYST. Maximum weekly doses of up to 320 mg have been administered subcutaneously for up to approximately 18 months in a small number of patients with CAPS and up to 6 months in patients with an unapproved indication in clinical trials without evidence of dose-limiting toxicities. In addition, ARCALYST given intravenously at doses up to 2000 mg monthly in another patient population for up to six months was tolerated without dose-limiting toxicities. The maximum amount of ARCALYST that can be safely administered has not been determined.
In case of overdose, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects, and appropriate symptomatic treatment instituted immediately.
5DESCRIPTION
Rilonacept is a dimeric fusion protein consisting of the ligand-binding domains of the extracellular portions of the human interleukin-1 receptor component (IL-1R1) and IL-1 receptor accessory protein (IL-1RAcP) linked in-line to the Fc portion of human IgG1. Rilonacept has a molecular weight of approximately 251 kDa. Rilonacept is expressed in recombinant Chinese hamster ovary (CHO) cells.
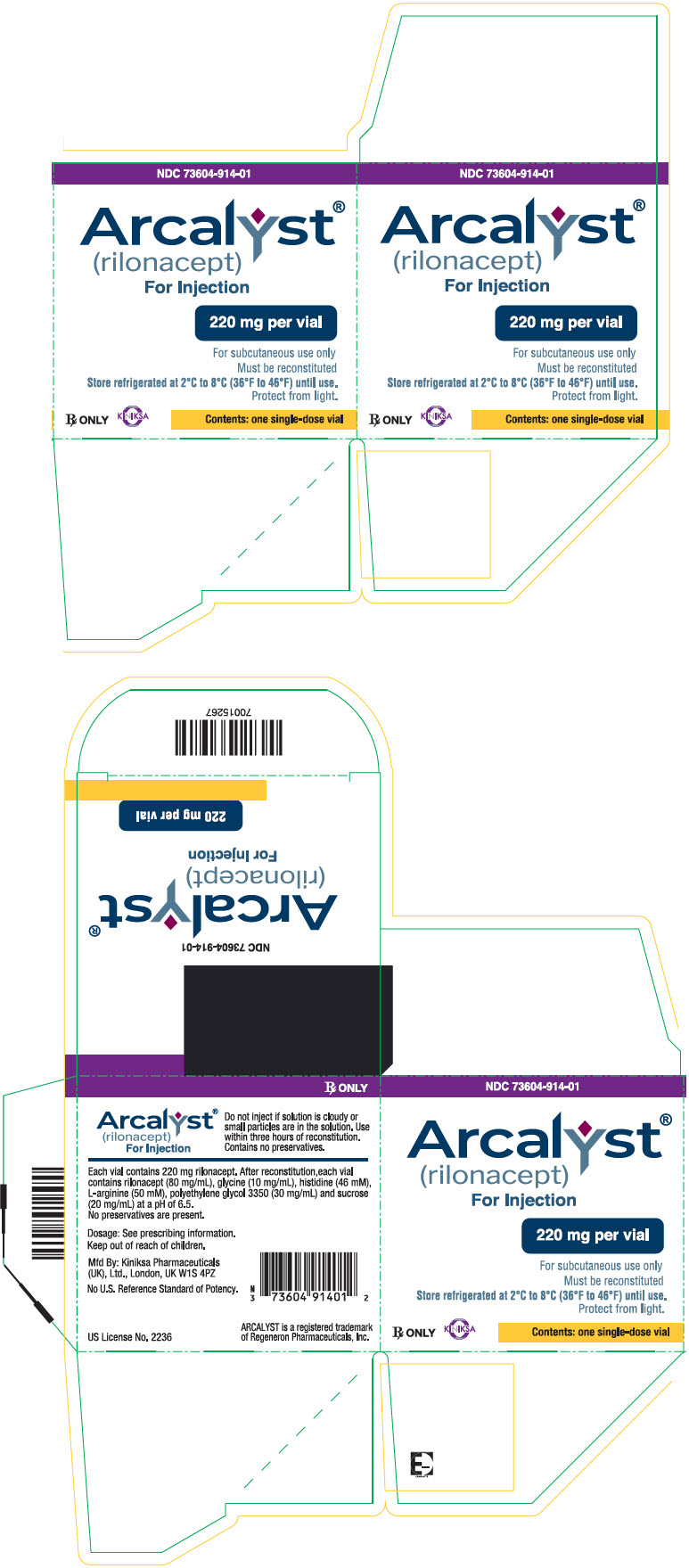
ARCALYST is supplied in single-dose vials containing a sterile, white to off-white lyophilized powder. Each vial of ARCALYST is to be reconstituted with 2.3 mL of Sterile Water for Injection, USP. A volume of up to 2 mL can be withdrawn, which is designed to deliver 160 mg for subcutaneous administration only. The resulting solution is viscous, clear, colorless to pale yellow, and essentially free from particulates. Each vial contains 220 mg rilonacept. After reconstitution, each vial contains rilonacept (80 mg/mL), glycine (10 mg/mL), histidine (46 mM), L-arginine (50 mM), polyethylene glycol 3350 (30 mg/mL), and sucrose (20 mg/mL) at a pH of 6.5. No preservatives are present.
6HOW SUPPLIED/STORAGE AND HANDLING
ARCALYST (rilonacept) for injection is supplied as a sterile, white to off-white, preservative-free, lyophilized powder in single-dose vials.
Each 220 mg vial of ARCALYST is supplied in a carton containing one vial (NDC 73604-914-01) or four vials (NDC 73604-914-04).
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Assessment of Patient's or Caregiver's Administration of ARCALYST: The first injection of ARCALYST should be performed under the supervision of a qualified healthcare professional. If a patient or caregiver is to administer ARCALYST, instruct them on aseptic reconstitution of the lyophilized product and injection technique. The ability to inject subcutaneously should be assessed to ensure proper administration of ARCALYST, including rotation of injection sites. (See ). ARCALYST should be reconstituted with preservative-free Sterile Water for Injection to be provided by the pharmacy. If the total volume of dose needed is greater than 2 mL, provide instruction about how to divide the total dose and how to administer the 2 injections. Remind patients to discard vials with unused product. A sharps disposal container should be used for disposal of vials, needles and syringes. Instruct patients or caregivers in proper vial, syringe, and needle disposal, and they should be cautioned against reuse of these items [see .
Injection-site Reactions: Explain to patients that almost half of the patients in the clinical trials experienced a reaction at the injection site. Injection-site reactions may include pain, erythema, swelling, pruritus, bruising, mass, inflammation, dermatitis, edema, urticaria, vesicles, warmth, and hemorrhage. Caution patients to avoid injecting into an area that is already swollen or red. Any persistent reaction should be brought to the attention of the prescribing physician [see .
Infections: Caution patients that ARCALYST has been associated with serious, life-threatening infections, and not to initiate treatment with ARCALYST if they have a chronic or active infection. Advise patients to contact their healthcare professional immediately if they develop an infection after starting ARCALYST. Treatment with ARCALYST should be discontinued if a patient develops a serious infection. Advise patients not to take any IL-1 blocking drug, including ARCALYST, if they are also taking a drug that blocks TNF such as etanercept, infliximab, or adalimumab. Use of ARCALYST with other IL-1 blocking agents, such as anakinra, is not recommended [see .
Vaccinations: Prior to initiation of therapy with ARCALYST, review the vaccination history with adult and pediatric patients, parent(s), and/or caregiver relative to current medical guidelines for vaccine use, including taking into account the potential of increased risk of infection during treatment with ARCALYST [see .
8Instructions for Use
ARCALYST
It is important that you read, understand, and follow these instructions before you or your child start using ARCALYST so that you prepare and inject the medicine the right way.
Do not try to inject ARCALYST until you have been shown the right way to give the injections by your or your child's healthcare provider.
How do I prepare and give an ARCALYST injection?
Step 1: Setting up for an injection
- Choose a table or other flat area to set up the supplies for your injection. Be sure the area is clean or clean it with an antiseptic or soap and water.
- Wash your hands well with soap and water about 20 seconds, and dry with a clean towel.
- Put the following supplies on the cleaned area for each injection (see
Supplies needed to give your ARCALYST injection:
- 1 vial of ARCALYST (powder for mixing)
Additional supplies needed (available from the pharmacy):
- 1 vial of preservative-free Sterile Water for Injection, USP
- 2 sterile, 3-milliliter (mL) disposable syringes with markings at each 0.1 mL (see
- 2 sterile disposable needles (18-gauge, 1-inch or 1½-inch) and 1 sterile disposable needle (26-gauge, ½-inch) with needle covers
- 4 alcohol wipes
- 1 gauze pad
- 1 sharps disposal container for throwing away (disposing of) used needles, syringes, and vials
Note:
- Do not use Sterile Water for Injection, USP, syringes or needles other than those provided by your pharmacy. Contact your pharmacy if you need replacement syringes or needles.
- Do not touch the needles or the rubber stoppers on the vials with your hands. If you touch the rubber stopper, clean it with a new alcohol wipe.
- If you touch a needle or the needle touches any surface, throw away the entire syringe in the sharps disposal container and use a new syringe.
- Do not reuse needles or syringes.
- To protect yourself and others from possible needlesticks, it is very important to throw away each syringe, with the needle attached, in the sharps disposal container right after use.
Step 2: Preparing the vials
- Check the expiration date on the carton or on the vial of ARCALYST. Do not use the vial if the expiration date has passed. Contact your pharmacy if the expiration date has passed.
- Check the expiration date on the vial of Sterile Water for Injection, USP. Do not use the vial if the expiration date has passed. Contact your pharmacy for assistance.
- Remove the protective plastic caps from both vials.
- Clean the top of each vial with an alcohol wipe. Use 1 wipe for each vial and wipe in 1 direction around the top of the vial (see
- Check the expiration date on the needle. Do not use the needle if the expiration date has passed. Contact your pharmacy if the expiration date has passed.
- Open the wrapper that contains 1 of the 18-gauge needles by pulling apart the tabs and set it aside for later use. Do not remove the needle cover. This needle will be used to mix the water with ARCALYST powder in the vial.
- Check the expiration date on the syringe. Do not use the syringe if the expiration date has passed. Contact your pharmacy if the expiration date has passed.
- Open the wrapper that contains the syringe by pulling apart the tabs (see
- Hold the barrel of the syringe with one hand and use your other hand to twist the 18-gauge needle with the cover onto the tip of the syringe until it fits firmly (see
- Hold the syringe upright at eye level. With the needle cover still on the 18-gauge needle, pull back the plunger to the 2.3 mL mark to fill the syringe with air (see
- Hold the syringe in one hand and use the other hand to pull the needle cover straight off. Do not twist the needle as you pull off the cover. Place the needle cover aside. Hold the syringe in the hand that you will use to mix your medicine. Hold the Sterile Water for Injection, USP, vial on a firm surface with your other hand. Slowly insert the needle straight through the rubber stopper. Do not bend the needle. Push the plunger in all the way to push the air into the vial (see
- Hold the vial in one hand and the syringe in the other hand and carefully turn the vial upside down so that the needle is pointing straight up (see
- Make sure the tip of the needle is covered by the liquid and slowly pull back on the plunger to the 2.3 mL mark to withdraw the Sterile Water for Injection, USP, from the vial (see
- Keep the vial upside down and gently tap the syringe with your fingers until any air bubbles rise to the top of the syringe.
- To remove the air bubbles, gently push in the plunger so only the air is pushed out of the syringe and back into the bottle.
- After removing the air bubbles, check the syringe to be sure that the right amount of Sterile Water for Injection, USP, has been drawn into the syringe (see
- Carefully remove the syringe with the 18-gauge needle from the Sterile Water for Injection, USP, vial. Do not touch the needle.
Step 3: Mixing ARCALYST
- With one hand, hold the ARCALYST vial on a firm surface.
- With the other hand, take the syringe with the 18-gauge needle that contains the Sterile Water for Injection, USP, and slowly insert the needle straight down through the rubber stopper of the ARCALYST vial.
- Gently push the plunger in all the way to inject the Sterile Water for Injection, USP, into the vial, aiming the stream of Sterile Water for Injection, USP, down the side of the vial into the powder (see
- Remove the syringe and needle from the rubber stopper and throw away the needle, syringe, and Sterile Water for Injection, USP, vial in the sharps disposal container. Do not try to put the needle cover back on the needle (see
- Hold the vial containing the ARCALYST and Sterile Water for Injection, USP, sideways (not upright) as shown (see
- Put the vial back on the table and let the vial sit for about 1 minute.
- Check the vial for any particles or clumps of powder that have not dissolved.
- If the powder has not completely dissolved, shake the vial quickly back and forth for 30 seconds more. Let the vial sit for about 1 minute.
- Repeat Step 25 until the powder is completely dissolved and the solution is clear (see
- The mixed ARCALYST should be thick, clear, and colorless to pale yellow. Do not use the mixed liquid if it is discolored or cloudy, or if particles are in it.
- ARCALYST may be kept at room temperature after mixing. ARCALYST should be used within
Step 4: Preparing the Injection
- Hold the ARCALYST vial in one hand and wipe in 1 direction around the top of the ARCALYST vial with a new alcohol wipe with the other hand (see
- Take a new sterile, disposable 18-gauge needle and attach it firmly to a new syringe without removing the needle cover (see
- To draw air into the syringe, hold the syringe upright at eye level. Do not remove the needle cover. Pull back the plunger on the syringe to the mark that is equal to the amount of mixed ARCALYST that the healthcare provider has prescribed for you to inject (see
- Hold the syringe in one hand and use the other hand to pull the needle cover straight off. Do not twist the needle as you pull off the cover. Place the needle cover aside and be careful not to touch the needle. Keep the ARCALYST vial on a flat firm surface and slowly insert the needle straight down through the rubber stopper. Push the plunger down and inject all of the air into the vial (see
- Hold the vial in one hand and the syringe in the other hand and carefully turn the vial upside down so that the needle is pointing straight up. Hold the vial at eye level.
- Keep the tip of the needle in the liquid and slowly pull back on the plunger to the mark on the syringe that matches the amount of medicine prescribed by your or your child's healthcare provider (see
- Keep the vial upside down with the needle straight up, and gently tap the syringe until any air bubbles rise to the top of the syringe (see
- To remove the air bubbles, slowly and gently push in the plunger so only the air is pushed through the needle.
- Check to make sure that you have the amount of medicine prescribed by the healthcare provider in the syringe. Remove the syringe with the needle from the vial.
- You will now prepare to switch needles.
- To remove the 18-gauge needle and replace it with the new 26-gauge needle for injection, place the syringe with the 18-gauge needle and the needle cap on a flat surface.
- When the needle is covered, push the needle cap toward the syringe to fully attach it
- Open a new sterile, disposable 26-gauge needle (see
- Throw away the ARCALYST vial and 18-gauge needle that still has the needle cover attached to it in the sharps disposal container even if there is medicine left in the vial (see
Step 5: Giving the Injection
- ARCALYST is given by injection into the tissue directly under the skin (subcutaneous injection). Do not inject ARCALYST into any muscle, vein, or artery.
- Choose the area for the injection. Clean the area in a circular motion with a new alcohol wipe. Begin at the center of the injection site and move outward. Let the alcohol air dry completely.
- Remove the needle cover and hold the syringe in one hand like you would hold a pencil.
- With the other hand, gently pinch a fold of skin at the cleaned injection site (see
- Use a quick "dart like" motion to insert the needle straight into the skin at a 90-degree angle (see
- After the needle is completely in the skin, let go of the pinched skin.
- With your free hand, hold the syringe near the bottom. Gently pull back the plunger. If blood comes into the syringe, the needle has entered a blood vessel. Remove the needle and throw away (discard) the syringe and needle in the sharps disposal container. Start over with "
- If no blood comes into the syringe, inject all the medicine in the syringe at a slow, steady rate, pushing the plunger all the way down. It may take up to 30 seconds to inject the entire dose.
- Pull the needle out of the skin and hold a gauze pad over the injection site for several seconds (see
- Do not replace the needle cover. Throw away the vials, used syringes and needles in an FDA-cleared sharps disposal container (see
- Keep the sharps disposal container out of the reach of children.
- Used alcohol wipes can be thrown away in the household trash.
Contact your or your child's healthcare provider right away with any questions or concerns about ARCALYST.
Manufactured by:
Kiniksa Pharmaceuticals (UK), Ltd., London, UK W1J 7NJ
U.S. License Number 2236
ARCALYST is a registered trademark of Regeneron Pharmaceuticals, Inc.
© 2025 Kiniksa Pharmaceuticals (UK), Ltd. All rights reserved.
For more information about ARCALYST, call 1-833-KINIKSA (1-833-546-4572), or visit www.ARCALYST.com.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: October 2025
9PRINCIPAL DISPLAY PANEL - 220 mg Vial Carton - NDC 73604-914-04
NDC 73604-914-04
Arcalyst
220 mg per vial
For subcutaneous use only
Rx ONLY

10PRINCIPAL DISPLAY PANEL - 220 mg Vial Carton - NDC 73604-914-01
NDC 73604-914-01
Arcalyst
220 mg per vial
For subcutaneous use only.
Rx ONLY

