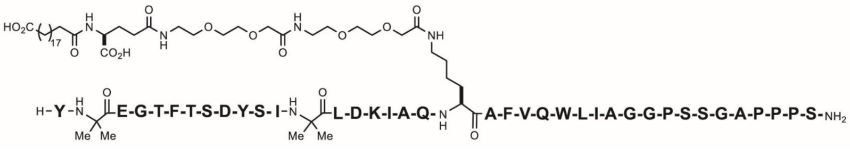
Tirzepatide
What is Zepbound (Tirzepatide)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this Phase-2 chronic weight management master protocol (CWMM) is to create a framework to evaluate the safety and efficacy of various investigational interventions for chronic weight management with intervention-specific appendices (ISAs). The CWMM establishes entry criteria for newly enrolled participants across the master and the ISAs. The ISAs may start independently of other ISA...
Summary: This phase II trial compares the effect of time-restricted eating (TRE) and glucagon-like peptide-1 (GLP1) receptor agonists (RA), semaglutide and tirzepatide, to an American Heart Association (AHA) heart healthy diet (HHD) intervention on heart and blood vessel health (cardiovascular system) and how the body processes food for energy (metabolic system) in prostate cancer patients undergoing andro...
Summary: The objective of this Phase 2 randomized controlled trial is to evaluate the effects of weekly tirzepatide (vs. placebo) on alcohol consumption and cardiometabolic outcomes in adults with alcohol use disorder and overweight or obesity.
Related Latest Advances
Brand Information
- In rats, tirzepatide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether ZEPBOUND causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of tirzepatide-induced rodent thyroid C-cell tumors has not been determined[see Warnings and Precautions (
- ZEPBOUND is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)[see Contraindications (. Counsel patients regarding the potential risk for MTC with the use of ZEPBOUND and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with ZEPBOUND[see Contraindications (
- to reduce excess body weight and maintain weight reduction long term in adults with obesity or adults with overweight in the presence of at least one weight-related comorbid condition.
- to treat moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
- 2.5 mg/0.5 mL
- 5 mg/0.5 mL
- 7.5 mg/0.5 mL
- 10 mg/0.5 mL
- 12.5 mg/0.5 mL
- 15 mg/0.5 mL
- A personal or family history of MTC or in patients with MEN 2
- Known serious hypersensitivity to tirzepatide or any of the excipients in ZEPBOUND. Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with tirzepatide
- Risk of Thyroid C-cell Tumors
- Severe Gastrointestinal Adverse Reactions
- Acute Kidney Injury Due to Volume Depletion
- Acute Gallbladder Disease
- Acute Pancreatitis
- Hypersensitivity Reactions
- Hypoglycemia
- Diabetic Retinopathy Complications in Patients with Type 2 Diabetes Mellitus
- Suicidal Behavior and Ideation
- Pulmonary Aspiration During General Anesthesia or Deep Sedation
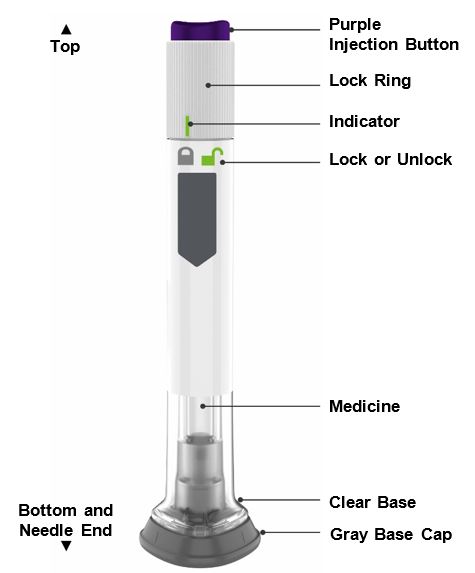
- ZEPBOUND is a single-dose prefilled pen.
- ZEPBOUND is used 1 time each week.
- Inject under the skin (subcutaneously) only.
- You or another person can inject into your stomach (abdomen) or thigh.
- Another person can inject into the back of your upper arm.
- Put your used pen in an FDA-cleared sharps disposal container right away after use.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not recycle your used sharps disposal container.
- Store your pen in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store your pen at room temperature up to 86°F (30°C) for up to 21 days. If you store the pen at room temperature, do not return the pen to the refrigerator.
- Discard the pen if not used within 21 days after removing from the refrigerator.
- Do not freeze your pen. If the pen has been frozen, throw the pen away and use a new pen.
- Store your pen in the original carton to protect your pen from light.
- The pen has glass parts. Handle it carefully. If you drop the pen on a hard surface,
- Keep your ZEPBOUND pen and all medicines out of the reach of children.
- If you have vision problems,
- If you have questions or problems with your ZEPBOUND pen, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) or call your healthcare provider.
- For more information about the ZEPBOUND pen, visit our website at www.zepbound.com.

- ZEPBOUND is a single-dose vial.
- ZEPBOUND is used 1 time each week.
- Inject under the skin (subcutaneously) only.
- You or another person may inject into your stomach (abdomen) or thigh.
- Another person can inject into the back of your upper arm.
- 1 single-dose ZEPBOUND vial
- 1 syringe and 1 needle, supplied separately (for example, use a 1 mL syringe and needle as recommended by your healthcare provider)
- 1 alcohol swab
- gauze
- 1 sharps container for throwing away used needles and syringes.
- Inject exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you should pinch the skin before injecting.
- Change (rotate) your injection site within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not mix ZEPBOUND with any other medicine.
- Do not inject ZEPBOUND in the same injection site used for other medicines.
- Put your used needle and syringe in an FDA-cleared sharps disposal container right away after use.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Store all unopened vials in the refrigerator at 36°F to 46°F (2°C to 8°C).
- You may store the unopened vial at room temperature up to 86°F (30°C) for up to 21 days.
- Do not freeze. Do not use if ZEPBOUND has been frozen.
- Store the vial in the original carton to protect from light.
- Throw away all opened vials after use, even if there is medicine left in the vial.
