Alendronate
What is Fosamax (Alendronate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this research is to identify strategies that minimize bone loss that occurs when older adults lose weight. Participation in this research will involve up to nine assessment visits and last up to two years.
Summary: In women with breast cancer undergoing adjuvant hormone therapy, the marked tissue hypoestrogenism induced by therapy with aromatase inhibitors and/or tamoxifen ± GnRH analogues causes a significant acceleration in bone mass loss, with a consequent increased risk of fracture from the first year of therapy. It is therefore essential to start treatment with antiresorptive drugs and calcium and vitam...
Summary: This study investigates the use of blood tests known as Bone Turnover Markers (BTMs) to quickly monitor the effectiveness of osteoporosis treatment in postmenopausal women. Osteoporosis, which weakens bones and increases fracture risk, is typically monitored using a DEXA scan to measure bone density (BMD), but this method changes slowly. BTMs may show a response to medication within just 3 to 6 mo...
Related Latest Advances
Brand Information
- 70 mg/2800 international units tablets are white to off-white, modified capsule-shaped tablets with code 710 on one side and an outline of a bone image on the other.
- 70 mg/5600 international units tablets are white to off-white, modified rectangle-shaped tablets with code 270 on one side and an outline of a bone image on the other.
- Abnormalities of the esophagus which delay esophageal emptying such as stricture or achalasia
- Inability to stand or sit upright for at least 30 minutes
- Hypocalcemia
- Hypersensitivity to any component of this product. Hypersensitivity reactions including urticaria and angioedema have been reported
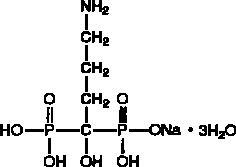
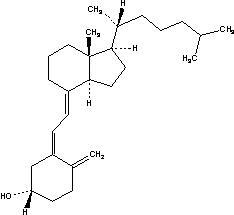
(alendronate sodium/
cholecalciferol) tablets
2800IU

(alendronate sodium/
cholecalciferol) tablets
5600IU




