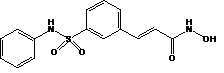
Beleodaq
What is Beleodaq (Belinostat)?
Approved To Treat
Related Clinical Trials
Background: High-grade neuroendocrine carcinomas (HGNEC) are cancers that develop in different parts of the body, including the digestive tract, genitals, neck, and head. One drug (belinostat), combined with 2 other drugs (etoposide and cisplatin), is approved to treat HGNEC. But some people may have a gene variant that affects how quickly their body gets rid of the drug; these people may do better with diffe...
Summary: This phase I trial tests the safety, side effects, and best dose of combination therapy with tazemetostat and belinostat in treating patients with lymphoma that has come back after a period of improvement (relapsed) or that does not respond to treatment (refractory). Tazemetostat is in a class of medications called EZH2 inhibitors. The EZH2 gene provides instructions for making a type of enzyme ca...
Summary: This phase I trial studies the side effects and best dose of belinostat when given together with durvalumab in treating patients with urothelial cancer that has spread to other places in the body (metastatic) or cannot be removed by surgery (unresectable) and has spread to nearby tissue or lymph nodes (locally advanced). Immunotherapy with monoclonal antibodies, such as durvalumab, may help the bo...
Related Latest Advances
Brand Information
- Hematologic Toxicity
- Infection
- Hepatotoxicity
- Tumor Lysis Syndrome
- Gastrointestinal Toxicity
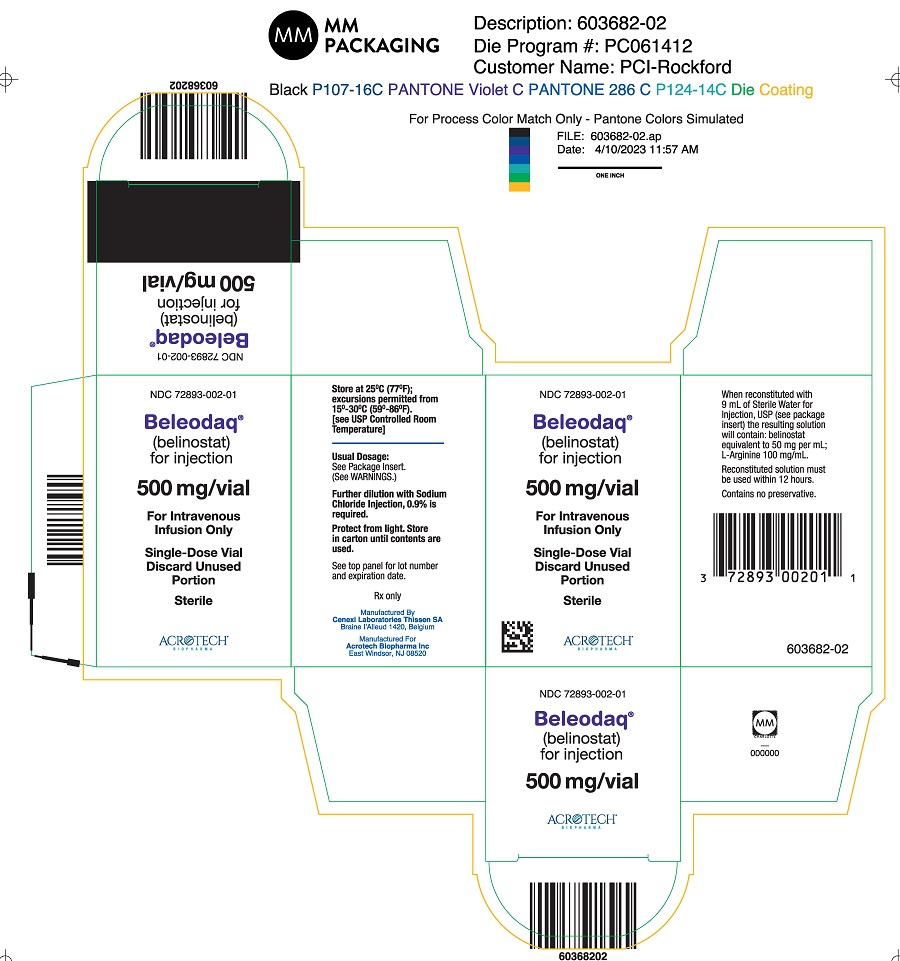
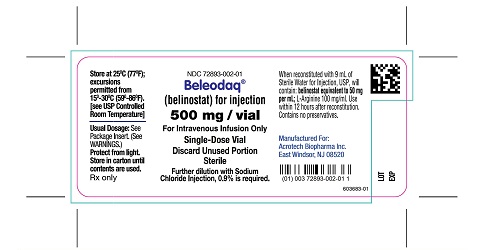
Beleodaq (belinostat) for injection is supplied in single vial cartons; each clear vial contains sterile, lyophilized powder equivalent to 500 mg belinostat.
NDC 72893-002-01: Individual carton of Beleodaq single-dose vial containing 500 mg belinostat.
- To report symptoms of nausea, vomiting and diarrhea so that appropriate antiemetic and antidiarrheal medications can be administered [see
- To report any symptoms of thrombocytopenia, leukopenia (neutropenia and lymphopenia), and anemia [see
- To immediately report symptoms of infection (e.g., pyrexia) [see
- Of the potential risk to the fetus and for women to avoid pregnancy and use effective contraception while receiving Beleodaq and for 6 months after the last dose [
- To avoid breastfeeding while receiving Beleodaq and for 2 weeks after the last dose [see
- To understand the importance of monitoring liver function test abnormalities and to immediately report potential symptoms of liver injury [see