Generic Name
Alendronate
Brand Names
Fosamax, Binosto
FDA approval date: February 06, 2008
Classification: Bisphosphonate
Form: Tablet, Solution
What is Fosamax (Alendronate)?
Alendronate sodium tablets are a bisphosphonate indicated for: Treatment and prevention of osteoporosis in postmenopausal women.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
FOSAMAX PLUS D (ALENDRONATE SODIUM and CHOLECALCIFEROL)
1DOSAGE FORMS AND STRENGTHS
- 70 mg/2800 international units tablets are white to off-white, modified capsule-shaped tablets with code 710 on one side and an outline of a bone image on the other.
- 70 mg/5600 international units tablets are white to off-white, modified rectangle-shaped tablets with code 270 on one side and an outline of a bone image on the other.
2CONTRAINDICATIONS
FOSAMAX PLUS D is contraindicated in patients with the following conditions:
- Abnormalities of the esophagus which delay esophageal emptying such as stricture or achalasia
- Inability to stand or sit upright for at least 30 minutes
- Hypocalcemia
- Hypersensitivity to any component of this product. Hypersensitivity reactions including urticaria and angioedema have been reported
3OVERDOSAGE
Alendronate Sodium
Significant lethality after single oral doses with alendronate was seen in female rats and mice at 552 mg/kg (3256 mg/m
No specific information is available on the treatment of overdosage with alendronate. Hypocalcemia, hypophosphatemia, and upper gastrointestinal adverse events, such as upset stomach, heartburn, esophagitis, gastritis, or ulcer, may result from oral overdosage. Milk or antacids should be given to bind alendronate. Due to the risk of esophageal irritation, vomiting should not be induced and the patient should remain fully upright.
Dialysis would not be beneficial.
Cholecalciferol
Significant lethality occurred in mice treated with a single high oral dose of calcitriol (4 mg/kg), the hormonal metabolite of cholecalciferol.
There is limited information regarding doses of cholecalciferol associated with acute toxicity, although intermittent (yearly or twice yearly) single doses of ergocalciferol (vitamin D
Dialysis to remove vitamin D would not be beneficial.
4DESCRIPTION
FOSAMAX PLUS D contains alendronate sodium, a bisphosphonate, and cholecalciferol (vitamin D
Alendronate sodium is a bisphosphonate that acts as a specific inhibitor of osteoclast-mediated bone resorption. Bisphosphonates are synthetic analogs of pyrophosphate that bind to the hydroxyapatite found in bone.
Alendronate sodium is chemically described as (4-amino-1-hydroxybutylidene) bisphosphonic acid monosodium salt trihydrate.
The empirical formula of alendronate sodium is C
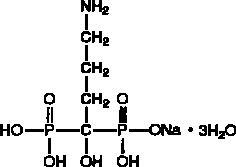
Alendronate sodium is a white, crystalline, nonhygroscopic powder. It is soluble in water, very slightly soluble in alcohol, and practically insoluble in chloroform.
Cholecalciferol (vitamin D
The chemical name of cholecalciferol is (3β,5
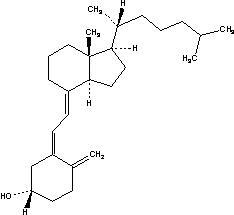
Cholecalciferol is a white, crystalline, odorless powder. Cholecalciferol is practically insoluble in water, freely soluble in usual organic solvents, and slightly soluble in vegetable oils.
FOSAMAX PLUS D for oral administration contains 91.37 mg of alendronate monosodium salt trihydrate, the molar equivalent of 70 mg of free acid, and 70 or 140 mcg of cholecalciferol, equivalent to 2800 or 5600 international units vitamin D, respectively. Each tablet contains the following inactive ingredients: microcrystalline cellulose, lactose anhydrous, medium chain triglycerides, gelatin, croscarmellose sodium, sucrose, colloidal silicon dioxide, magnesium stearate, butylated hydroxytoluene, modified food starch, and sodium aluminum silicate.
5HOW SUPPLIED/STORAGE AND HANDLING
FOSAMAX PLUS D 70 mg/2800 international units are white to off-white, modified capsule-shaped tablets with code 710 on one side and an outline of a bone image on the other. They are supplied as follows:
NDC 78206-137-01 unit of use blister packages of 4.
FOSAMAX PLUS D 70 mg/5600 international units are white to off-white, modified rectangle-shaped tablets with code 270 on one side and an outline of a bone image on the other. They are supplied as follows:
NDC 78206-136-01 unit of use blister packages of 4
Storage
Store at 20-25°C (68-77°F), excursions between 15-30°C (59-86°F) are allowed. [See USP Controlled Room Temperature.] Protect from moisture and light. Store tablets in the original blister package until use.
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Instruct patients to read the Medication Guide before starting therapy with FOSAMAX PLUS D and to reread it each time the prescription is renewed.
6.1Osteoporosis Recommendations, Including Calcium and Vitamin D Supplementation
Instruct patients to take supplemental calcium if intake is inadequate. Patients at increased risk for vitamin D insufficiency (e.g., over the age of 70 years, nursing home bound, or chronically ill) should take additional vitamin D if needed
6.2Dosing Instructions
Instruct patients that the expected benefits of FOSAMAX PLUS D may only be obtained when it is taken with plain water the first thing upon arising for the day at least 30 minutes before the first food, beverage, or medication of the day. Even dosing with orange juice or coffee has been shown to markedly reduce the absorption of alendronate
Instruct patients not to chew or suck on the tablet because of a potential for oropharyngeal ulceration.
Instruct patients to swallow each tablet of FOSAMAX PLUS D with a full glass of water (6-8 ounces)
Instruct patients not to take FOSAMAX PLUS D at bedtime or before arising for the day. Patients should be informed that failure to follow these instructions may increase their risk of esophageal problems.
Instruct patients that if they develop symptoms of esophageal disease (such as difficulty or pain upon swallowing, retrosternal pain or new or worsening heartburn) they should stop taking FOSAMAX PLUS D and consult their physician.
If patients miss a dose of FOSAMAX PLUS D, instruct patients to take one tablet on the morning after they remember. They should not take two tablets on the same day but should return to taking one tablet once a week, as originally scheduled on their chosen day.
7PRINCIPAL DISPLAY PANEL - 70 mg/2800 IU Tablet Blister Pack
FOSAMAX
(alendronate sodium/
cholecalciferol) tablets
(alendronate sodium/
cholecalciferol) tablets
70mg
2800IU
2800IU
Dispense the enclosed
Each tablet contains
710
4 Tablets
Rx only
USUAL ADULT DOSAGE:
See accompanying circular
Manuf. for: Organon LLC,
By:
Made in Spain

8PRINCIPAL DISPLAY PANEL - 70 mg/5600 IU Tablet Blister Pack
FOSAMAX
(alendronate sodium/
cholecalciferol) tablets
(alendronate sodium/
cholecalciferol) tablets
70mg
5600IU
5600IU
Dispense the enclosed
Each tablet contains
270
4 Tablets
Rx only
USUAL ADULT DOSAGE:
See accompanying circular
Manuf. for: Organon LLC,
By:
Made in Spain
