Brand Name
Evista
Generic Name
Raloxifene
View Brand Information FDA approval date: January 06, 1998
Classification: Estrogen Agonist/Antagonist
Form: Tablet
What is Evista (Raloxifene)?
Raloxifene hydrochloride tablets are an estrogen agonist/antagonist indicated for: Treatment and prevention of osteoporosis in postmenopausal women.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Evista (Raloxifene hydrochloride)
WARNING: INCREASED RISK OF VENOUS THROMBOEMBOLISM AND DEATH FROM STROKE
- Increased risk of deep vein thrombosis and pulmonary embolism have been reported with EVISTA[see Warnings and Precautions (. Women with active or past history of venous thromboembolism should not take EVISTA[see Contraindications (
- Increased risk of death due to stroke occurred in a trial in postmenopausal women with documented coronary heart disease or at increased risk for major coronary events. Consider risk-benefit balance in women at risk for stroke[see Warnings and Precautions (
1DOSAGE FORMS AND STRENGTHS
60 mg, white, elliptical, film-coated tablets (not scored) imprinted with 4165 on one side in edible blue ink.
2OVERDOSAGE
In an 8-week study of 63 postmenopausal women, a dose of raloxifene hydrochloride (HCl) 600 mg/day was safely tolerated. In clinical trials, no raloxifene overdose has been reported.
In postmarketing spontaneous reports, raloxifene overdose has been reported very rarely (less than 1 out of 10,000 [<0.01%] patients treated). The highest overdose has been approximately 1.5 grams. No fatalities associated with raloxifene overdose have been reported. Adverse reactions were reported in approximately half of the adults who took ≥180 mg raloxifene HCl and included leg cramps and dizziness.
Two 18-month-old children each ingested raloxifene HCl 180 mg. In these two children, symptoms reported included ataxia, dizziness, vomiting, rash, diarrhea, tremor, and flushing, as well as elevation in alkaline phosphatase.
There is no specific antidote for raloxifene.
No mortality was seen after a single oral dose in rats or mice at 5000 mg/kg (810 times the human dose for rats and 405 times the human dose for mice based on surface area, mg/m
3DESCRIPTION
EVISTA (raloxifene hydrochloride) is an estrogen agonist/antagonist, commonly referred to as a selective estrogen receptor modulator (SERM) that belongs to the benzothiophene class of compounds. The chemical structure is:
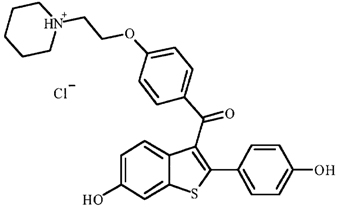
The chemical designation is methanone, [6-hydroxy-2-(4-hydroxyphenyl)benzo[
EVISTA is supplied in a tablet dosage form for oral administration. Each EVISTA tablet contains 60 mg of raloxifene HCl, which is the molar equivalent of 55.71 mg of free base. Inactive ingredients include anhydrous lactose, carnauba wax, crospovidone, FD&C Blue No. 2 aluminum lake, hypromellose, lactose monohydrate, magnesium stearate, modified pharmaceutical glaze, polyethylene glycol, polysorbate 80, povidone, propylene glycol, and titanium dioxide.
4PATIENT COUNSELING INFORMATION
See FDA-approved Medication Guide.
Physicians should instruct their patients to read the Medication Guide before starting therapy with EVISTA and to reread it each time the prescription is renewed.
4.1Osteoporosis Recommendations, Including Calcium and Vitamin D Supplementation
For osteoporosis treatment or prevention, patients should be instructed to take supplemental calcium and/or vitamin D if intake is inadequate. Patients at increased risk for vitamin D insufficiency (e.g., over the age of 70 years, nursing home bound, chronically ill, or with gastrointestinal malabsorption syndromes) should be instructed to take additional vitamin D if needed. Weight-bearing exercises should be considered along with the modification of certain behavioral factors, such as cigarette smoking and/or excessive alcohol consumption, if these factors exist.
4.2Patient Immobilization
EVISTA should be discontinued at least 72 hours prior to and during prolonged immobilization (e.g., post-surgical recovery, prolonged bed rest), and patients should be advised to avoid prolonged restrictions of movement during travel because of the increased risk of venous thromboembolic events
4.3Hot Flashes or Flushes
EVISTA may increase the incidence of hot flashes and is not effective in reducing hot flashes or flushes associated with estrogen deficiency. In some asymptomatic patients, hot flashes may occur upon beginning EVISTA therapy.
4.4Reduction in Risk of Invasive Breast Cancer in Postmenopausal Women with Osteoporosis or at High Risk of Invasive Breast Cancer
Use of EVISTA is associated with the reduction of the risk of invasive breast cancer in postmenopausal women. EVISTA has not been shown to reduce the risk of noninvasive breast cancer. When considering treatment, physicians need to discuss the potential benefits and risks of EVISTA treatment with the patient.
EVISTA is not indicated for the treatment of invasive breast cancer or reduction of the risk of recurrence.
Patients should have breast exams and mammograms before starting EVISTA and should continue regular breast exams and mammograms in keeping with good medical practice after beginning treatment with EVISTA.