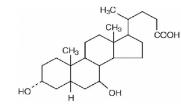
Ursodiol
What is Urso 250 (Ursodiol)?
Approved To Treat
Related Clinical Trials
Summary: The objective of this clinical trial is to evaluate the efficacy and safety of CnU capsule 750 mg administration in patients with Cholesterol gallstone (radiolucent gallstones)
Summary: The purpose of this clinical trial is to understand whether the drug Ursodeoxycholic acid (UDCA) can prevent glucose intolerance in participants with hyperlipidemia who are taking statins. It will also assess the safety of UDCA. The primary questions it aims to answer are: * Will UDCA reduce the incidence of glucose intolerance in participants taking oral statins? * Will the use of UDCA decrease o...
Summary: Primary biliary cholangitis (PBC) is an autoimmune liver disease predominantly affecting middle-aged women. While historically it was deemed rare, advancements in specific auto-antibody tests have led to increased recognition of PBC. The long-term survival of PBC patients in China is not yet fully understood. Several studies have investigated the prognosis of PBC in China. While these studies prov...
Related Latest Advances
Brand Information
- URSO 250: 250 mg tablet
- URSO Forte: 500 mg scored tablet
Allergan USA, Inc.
Madison, NJ 07940
www.allergan.com

NDC 58914-785-10
100 tablets
Rx only
Urso® 250
(Ursodiol Tablets, USP) 250 mg
Allergan™

NDC 58914-790-10
100 tablets
Urso Forte®
(Ursodiol Tablets, USP)
500 mg
Rx only
For the treatment of
Primary Biliary Cirrhosis
Allergan™



