The efficacy of XTANDI in patients with CRPC (N = 4692), mCSPC (N = 1150), or nmCSPC with high‑risk BCR (N = 1068) was demonstrated in six randomized, multicenter clinical trials. Patients received concomitant GnRH therapy or had prior bilateral orchiectomy, unless otherwise indicated.
AFFIRM (NCT00974311): XTANDI versus Placebo in Metastatic CRPC Following Chemotherapy
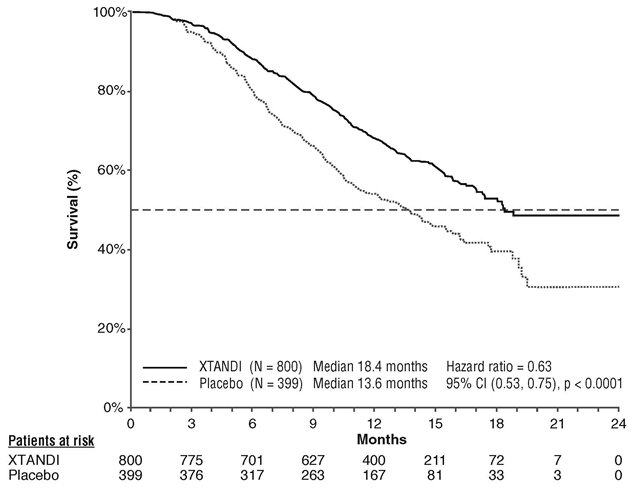
In AFFIRM, a total of 1199 patients who had received prior docetaxel-based chemotherapy were randomized 2:1 to receive either XTANDI orally at a dose of 160 mg once daily (N = 800) or placebo orally once daily (N = 399). Study treatment continued until disease progression (evidence of radiographic progression, a skeletal-related event, or clinical progression), initiation of new systemic antineoplastic treatment, unacceptable toxicity, or withdrawal. Patients with a previous history of seizure, taking medicines known to decrease the seizure threshold, or with other risk factors for seizure were not eligible
The following patient demographics and baseline disease characteristics were balanced between the treatment arms. The median age was 69 years (range 41-92) and the racial distribution was 92.7% White, 3.9% Black, 1.1% Asian, and 2.1% Other. Ninety-two percent of patients had an ECOG performance status score of 0-1 and 28% had a mean Brief Pain Inventory score of ≥ 4. Ninety-one percent of patients had metastases in bone and 23% had visceral involvement in the lung and/or liver. Fifty-nine percent of patients had radiographic evidence of disease progression and 41% had PSA-only progression on study entry. All patients had received prior docetaxel-based therapy and 24% had received two cytotoxic chemotherapy regimens. During the trial, 48% of patients on the XTANDI arm and 46% of patients on the placebo arm received glucocorticoids.
A statistically significant improvement in overall survival was demonstrated at the pre-specified interim analysis at the time of 520 deaths in patients on the XTANDI arm compared to patients on the placebo arm (
PREVAIL (NCT01212991): XTANDI versus Placebo in Chemotherapy-naïve Metastatic CRPC
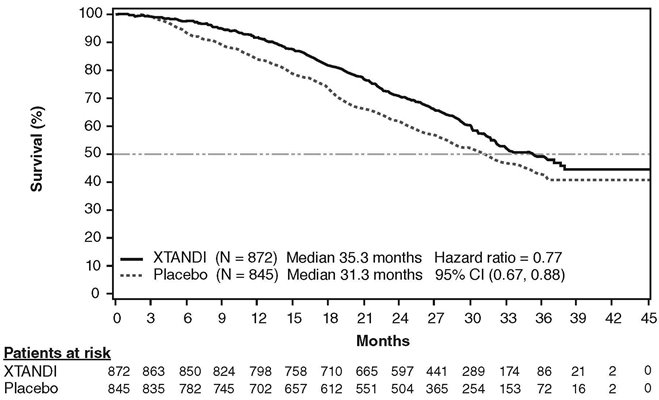
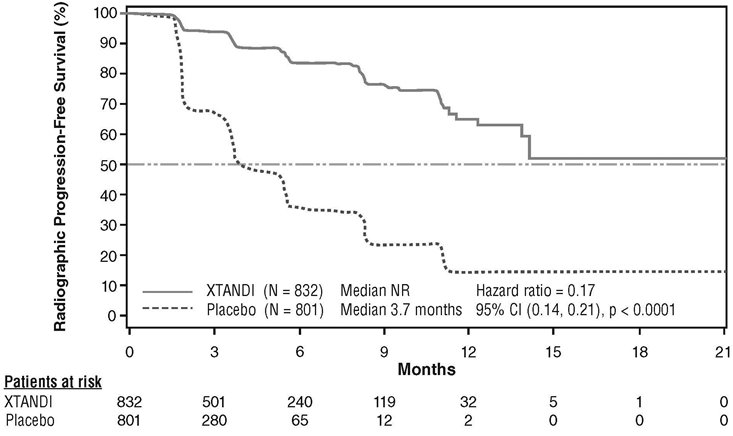
In PREVAIL, 1717 chemotherapy-naïve patients were randomized 1:1 to receive either XTANDI orally at a dose of 160 mg once daily (N = 872) or placebo orally once daily (N = 845). Patients with visceral metastases, patients with a history of mild to moderate heart failure (NYHA class I or II), and patients taking medications associated with lowering the seizure threshold were allowed. Patients with a previous history of seizure or a condition that might predispose to seizure and patients with moderate or severe pain from prostate cancer were excluded. Study treatment continued until disease progression (evidence of radiographic progression, a skeletal-related event, or clinical progression) and the initiation of a cytotoxic chemotherapy or an investigational agent, unacceptable toxicity, or withdrawal. Overall survival and radiographic progression-free survival (rPFS) were assessed. Radiographic progression was assessed with the use of sequential imaging and was defined by bone scan identification of 2 or more new bone lesions with confirmation (Prostate Cancer Clinical Trials Working Group 2 criteria) and/or Response Evaluation Criteria in Solid Tumors (RECIST v 1.1) criteria for progression of soft tissue lesions. The primary analysis of rPFS utilized centrally reviewed radiographic assessment of progression.
Patient demographics and baseline disease characteristics were balanced between the treatment arms at entry. The median age was 71 years (range 42-93) and the racial distribution was 77% White, 10% Asian, 2% Black and 11% Other. The ECOG performance status score was 0 for 68% of patients, and 1 for 32% of patients. Baseline pain assessment was 0-1 (asymptomatic) in 67% of patients, and 2-3 (mildly symptomatic) in 32% of patients as defined by the Brief Pain Inventory Short Form (worst pain over past 24 hours at study entry). Fifty-four percent of patients had radiographic evidence of disease progression and 43% had PSA-only progression. Twelve percent of patients had visceral (lung and/or liver) disease involvement. During the study, 27% of patients on the XTANDI arm and 30% of patients on the placebo arm received glucocorticoids for varying reasons.
A statistically significant improvement in overall survival was demonstrated at the pre-specified interim analysis, conducted after 540 deaths, in patients treated with XTANDI compared to those treated with placebo (
A statistically significant improvement in rPFS was demonstrated in patients treated with XTANDI compared to patients treated with placebo (
Time to initiation of cytotoxic chemotherapy was prolonged after XTANDI treatment, with a median of 28.0 months for patients on the XTANDI arm versus a median of 10.8 months for patients on the placebo arm [HR = 0.35 (95% CI: 0.30, 0.40), p < 0.0001].
The median time to first skeletal‑related event was 31.1 months for patients on the XTANDI arm versus 31.3 months for patients on the placebo arm [HR = 0.72 (95% CI: 0.61, 0.84), p < 0.0001]. A skeletal‑related event was defined as radiation therapy or surgery to bone for prostate cancer, pathologic bone fracture, spinal cord compression, or change of antineoplastic therapy to treat bone pain.
TERRAIN (NCT01288911): XTANDI versus Bicalutamide in Chemotherapy-naïve Metastatic CRPC
TERRAIN was conducted in 375 chemotherapy-naïve patients who were randomized 1:1 to receive either XTANDI orally at a dose of 160 mg once daily (N = 184) or bicalutamide orally at a dose of 50 mg once daily (N = 191). Patients with a previous history of seizure or a condition that might predispose to seizure and patients with moderate to severe pain from prostate cancer were excluded. Patients could have received prior bicalutamide, but those whose disease had progressed on prior antiandrogen therapy (e.g., bicalutamide) were excluded. Study treatment continued until disease progression (evidence of radiographic progression, a skeletal-related event), the initiation of subsequent antineoplastic agent, unacceptable toxicity, or withdrawal. Radiographic disease progression was assessed by Independent Central Review (ICR) using the Prostate Cancer Clinical Trials Working Group 2 criteria and/or Response Evaluation Criteria in Solid Tumors (RECIST v 1.1) criteria for progression of soft tissue lesions. Radiographic progression-free survival (rPFS) was defined as the time from randomization to the first objective evidence of radiographic progression as assessed by ICR or death, whichever occurred first.
Patient demographics and baseline disease characteristics were balanced between the treatment arms at entry. The median age was 71 years (range 48-96) and the racial distribution was 93% White, 5% Black, 1% Asian and 1% Other. The ECOG performance status score was 0 for 74% of patients and 1 for 26% of patients. Baseline pain assessment was 0‑1 (asymptomatic) in 58% of patients, and 2‑3 (mildly symptomatic) in 36% of patients as defined by the Brief Pain Inventory Short Form Question 3 (worst pain over past 24 hours at study entry). Ninety-eight percent of patients had objective evidence of disease progression at study entry. Forty-six percent of patients had received prior treatment with bicalutamide while no patients received prior treatment with XTANDI.
An improvement in rPFS was demonstrated in patients treated with XTANDI compared to patients treated with bicalutamide (

PROSPER (NCT02003924): XTANDI versus Placebo in Non-metastatic CRPC
PROSPER enrolled 1401 patients with non-metastatic CRPC who were randomized 2:1 to receive either XTANDI orally at a dose of 160 mg once daily (N = 933) or placebo orally once daily (N = 468). All patients in the PROSPER trial received a gonadotropin-releasing hormone (GnRH) analog or had a prior bilateral orchiectomy. Patients were stratified by Prostate Specific Antigen (PSA) Doubling Time (PSADT) and the use of bone-targeting agents. Patients were required to have a PSA doubling time ≤ 10 months, PSA ≥ 2 ng/mL, and confirmation of non-metastatic disease by blinded independent central review (BICR). PSA results were blinded and were not used for treatment discontinuation. Patients randomized to either arm discontinued treatment for radiographic disease progression confirmed by BICR, initiation of new treatment, unacceptable toxicity, or withdrawal.
The following patient demographics and baseline characteristics were balanced between the two treatment arms. The median age at randomization was 74 years (range 50-95) and 23% were 80 years of age or older. The racial distribution was 71% White, 16% Asian, and 2% Black. A majority of patients had a Gleason score of 7 or higher (77%). The median PSADT was 3.7 months. Fifty-four percent (54%) of patients received prior treatment for prostate cancer with either surgery or radiation. Sixty-three percent (63%) of patients received prior treatment with an anti-androgen; 56% of patients received bicalutamide and 11% of patients received flutamide. All patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1 at study entry.
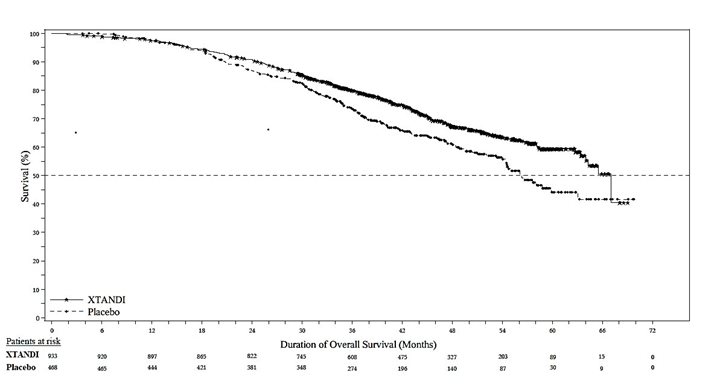
The major efficacy outcome of the study was metastasis-free survival (MFS), defined as the time from randomization to whichever of the following occurred first 1) loco-regional and/or distant radiographic progression per BICR or 2) death up to 112 days after treatment discontinuation without evidence of radiographic progression. A statistically significant improvement in MFS and OS was demonstrated in patients randomized to receive XTANDI compared with patients randomized to receive placebo. Consistent MFS results were observed when considering only distant radiographic progression events or deaths regardless of the cut-off date. Consistent MFS results were also observed in pre-specified and stratified patient sub-groups of PSADT (< 6 months or ≥ 6 months) and use of a prior bone-targeting agent (yes or no). The efficacy results from PROSPER are summarized in
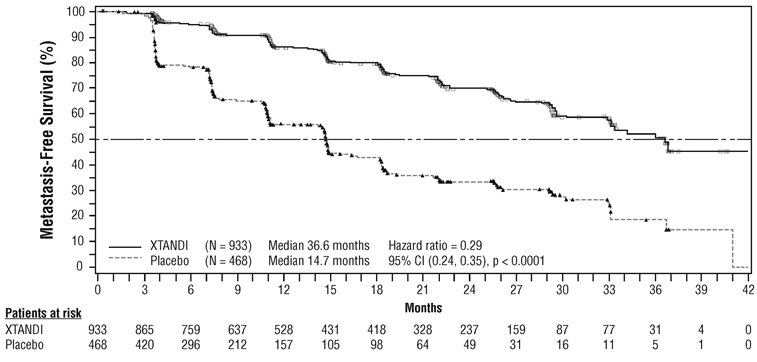
The primary efficacy outcome was also supported by a statistically significant delay in time to first use of new antineoplastic therapy (TTA) for patients in the XTANDI arm compared to those in the placebo arm. The median TTA was 39.6 months for patients on XTANDI and was 17.7 months for patients on placebo (HR = 0.21; 95% CI: [0.17, 0.26], p < 0.0001).
ARCHES (NCT02677896): XTANDI versus Placebo in Metastatic CSPC
ARCHES enrolled 1150 patients with mCSPC who were randomized 1:1 to receive XTANDI orally at a dose of 160 mg once daily (N = 574) or placebo orally once daily (N = 576). All patients in the trial received a GnRH analog or had a prior bilateral orchiectomy. Patients were stratified by volume of disease (low vs high) and prior docetaxel therapy for prostate cancer (no prior docetaxel, 1-5 cycles, or 6 prior cycles). High volume of disease is defined as metastases involving the viscera or, in the absence of visceral lesions, there must be 4 or more bone lesions, at least 1 of which must be in a bony structure beyond the vertebral column and pelvic bone. Treatment with concurrent docetaxel was not allowed. Patients continued treatment until radiographic disease progression, initiation of new treatment, unacceptable toxicity, or withdrawal.
The following patient demographics and baseline characteristics were balanced between the two treatment arms. The median age at randomization was 70 years (range: 42-92) and 30% were 75 years of age or older. The racial distribution was 81% White, 14% Asian, and 1% Black. Sixty-six percent (66%) of patients had a Gleason score of ≥ 8. Thirty-seven percent (37%) of patients had a low volume of disease and 63% of patients had a high volume of disease. Eighty-two percent (82%) of patients had no prior docetaxel treatment; 2% of patients had 1 to 5 cycles of docetaxel and 16% of patients had 6 prior cycles of docetaxel treatment. Twelve percent (12%) of patients received concomitant bone-targeted agents (bisphosphonates or RANKL inhibitors) which included both prostate and non-prostate cancer indications. The Eastern Cooperative Oncology Group Performance Status (ECOG PS) score was 0 for 78% of patients and 1 for 22% of patients at study entry.
The major efficacy outcome measure was radiographic progression-free survival (rPFS) based on blinded independent central review (BICR). Radiographic progression-free survival was defined as the time from randomization to radiographic disease progression at any time or death within 24 weeks after study drug discontinuation. Radiographic disease progression was defined by identification of 2 or more new bone lesions on a bone scan with confirmation (Prostate Cancer Working Group 2 criteria) and/or progression in soft tissue disease. Time to new antineoplastic therapy and OS were additional efficacy endpoints.
XTANDI demonstrated a statistically significant improvement in rPFS and OS compared to placebo. Consistent rPFS results were observed in patients with high or low volume of disease and patients with and without prior docetaxel therapy. Efficacy results for rPFS and OS from ARCHES are summarized in
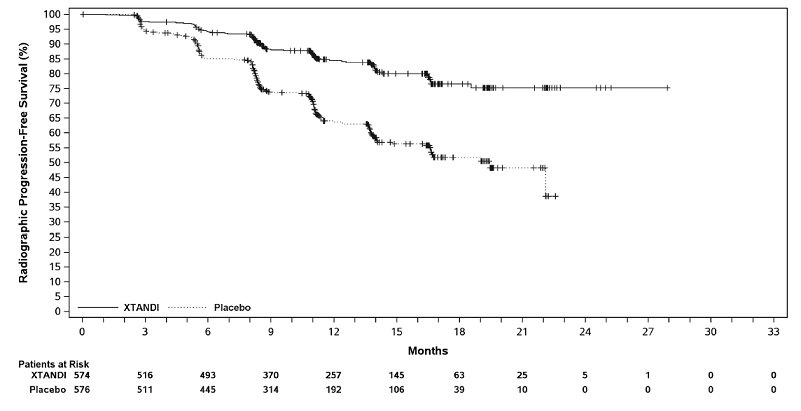
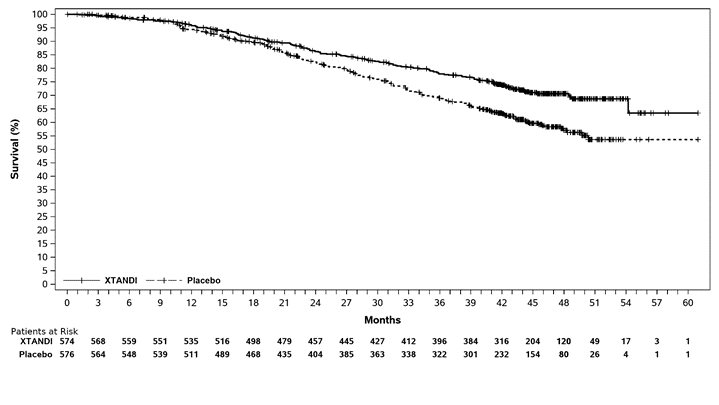
A statistically significant improvement was also reported on the XTANDI arm compared to placebo in time to initiation of a new antineoplastic therapy (HR = 0.28 [95% CI: 0.20, 0.40]; p < 0.0001).
EMBARK (NCT02319837): XTANDI versus Placebo in Non-metastatic CSPC with High-Risk BCR
EMBARK enrolled 1068 patients with nmCSPC with high-risk BCR who were randomized 1:1:1 to receive XTANDI orally at a dose of 160 mg once daily concurrently with leuprolide (N = 355), XTANDI orally at a dose of 160 mg once daily as open‑label as a single agent (N = 355), or placebo orally once daily concurrently with leuprolide (N = 358). All patients had prior definitive therapy with radical prostatectomy or radiotherapy (including brachytherapy) with curative intent, or both. Patients were not candidates for salvage radiotherapy at the time of enrollment. Patients were required to have confirmation of non-metastatic disease by BICR, high‑risk BCR (defined by a PSA doubling time ≤ 9 months), and PSA values ≥1 ng/mL if they had prior radical prostatectomy (with or without radiotherapy) as the primary treatment for prostate cancer or PSA values at least 2 ng/mL above the nadir if they had prior radiotherapy only.
Patients were stratified by screening PSA (≤10 ng/mL vs. >10 ng/mL), PSA doubling time (≤3 months versus >3 months to ≤ 9 months), and prior hormonal therapy. For patients whose PSA values were undetectable (<0.2 ng/mL) at week 36, treatment was suspended at week 37 and then reinitiated when PSA values increased to ≥2.0 ng/mL for patients with prior prostatectomy or ≥5.0 ng/mL for patients without prior prostatectomy. For patients whose PSA values were detectable (≥0.2 ng/mL) at week 36, treatment continued without suspension until permanent treatment discontinuation criteria were met. For all patients, treatment was permanently discontinued upon radiographic disease progression confirmed by BICR, initiation of new treatment, unacceptable toxicity, or withdrawal.
The median age at randomization was 69 years (range: 49-93) and 23% were 75 years of age or older. The racial distribution was 83% White, 7% Asian, 4% Black, 2.3% Others, and 2.7% not reported; 5.5% of patients were Hispanic or Latino. The median PSADT was 4.9 months. Seventy-four percent (74%) of patients had prior definitive therapy with radical prostatectomy, 34% of patients had prior primary radiotherapy (including brachytherapy), and 49% of patients had prior therapy with both surgery and radiotherapy (including adjuvant and salvage radiotherapy). Thirty-two percent (32%) of patients had a Gleason score of ≥ 8. The ECOG PS score was 0 for 92% of patients and 1 for 8% of patients at study entry.
The major efficacy outcome measure was metastasis-free survival (MFS) in patients randomized to receive XTANDI plus leuprolide compared to patients randomized to receive placebo plus leuprolide. MFS was defined as the time from randomization to whichever of the following occurred first 1) radiographic progression per BICR or 2) death. MFS in patients randomized to receive XTANDI as a single agent compared to patients randomized to receive placebo plus leuprolide and overall survival (OS) were additional efficacy outcome measures.
A statistically significant improvement in MFS was demonstrated in patients randomized to receive XTANDI plus leuprolide compared with patients randomized to receive placebo plus leuprolide. A statistically significant improvement in MFS was also demonstrated in patients randomized to receive XTANDI as a single agent compared with patients randomized to receive placebo plus leuprolide. The results are summarized in
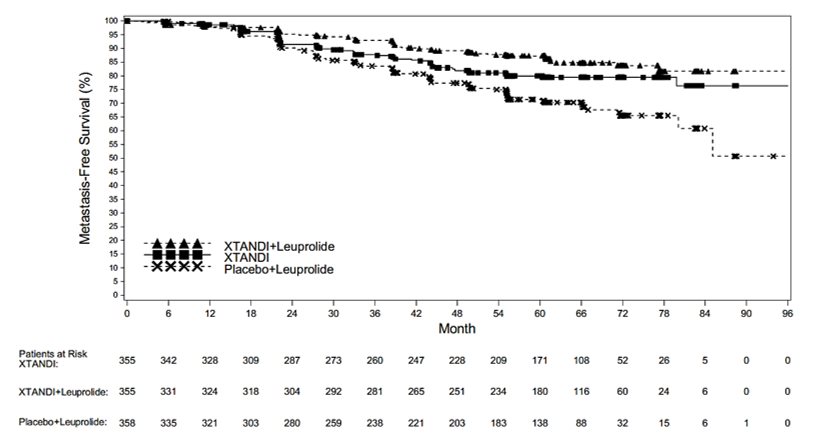
OS data were not mature at the time of MFS analysis (12.2% deaths across the overall population of 1068 patients).