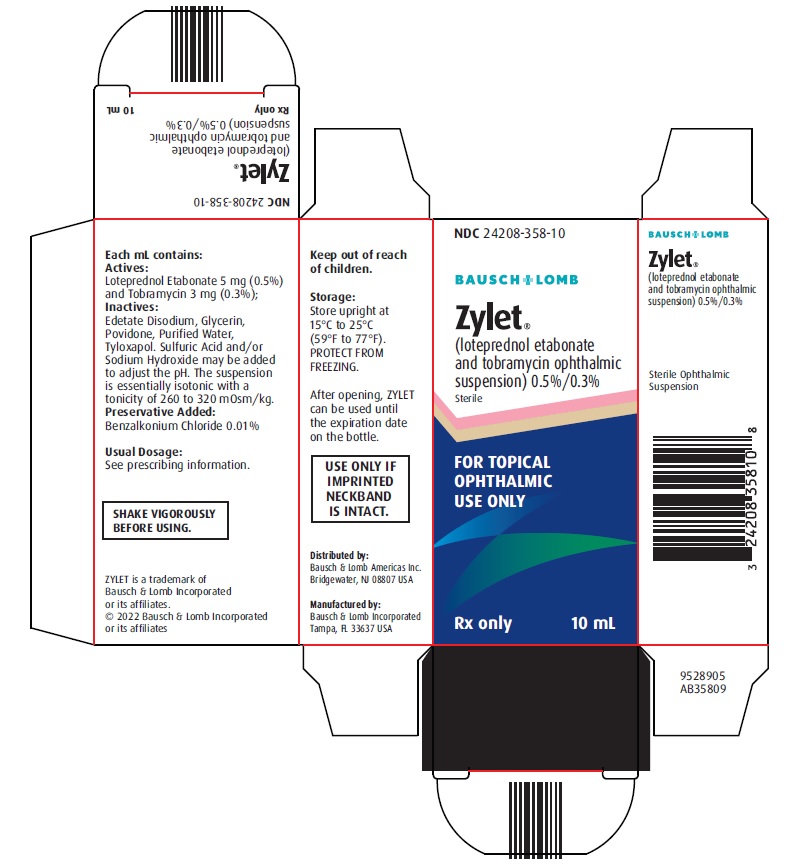
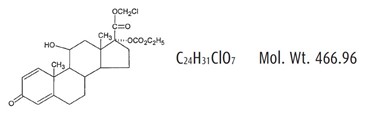
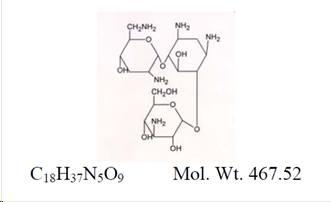
Loteprednol Etabonate
What is Zylet (Loteprednol Etabonate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The goal of this clinical trial is to learn if oral treatment with pivmecillinam is effective to treat febrile urinary tract infections in adult patients. Hospitalized patients who have received 2-4 days of intravenous antibiotic therapy for febrile urinary tract infections, and have responded to treatment, will be randomized to either pivmecillinam or standard treatment (other oral or intravenous...
Summary: The Beads vs Vac trial is a multi-centre randomized controlled trial of 312 participants with a severe open tibia fracture requiring multiple irrigation and debridement surgeries. Eligible participants will be randomized to receive either an antibiotic bead pouch or negative pressure wound therapy (NPWT) for their temporary open fracture wound management. Outcomes will be assessed at 6 weeks, 3 mo...
Summary: The goal of this randomized clinical trial is to study the best treatment for open lower leg fractures to prevent infection. The main questions it aims to answer is if treating tibia fracture patients with a calcium sulfate antibiotic depot is better at preventing infection that the standard of care.
Related Latest Advances
Brand Information
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
ZYLET and LOTEMAX are trademarks of Bausch & Lomb Incorporated or its affiliates.
BAUSCH + LOMB
Zylet®
(loteprednol etabonate
and tobramycin ophthalmic
suspension) 0.5%/0.3%
Sterile
OPHTHALMIC
USE ONLY
Rx only