Gattex
What is Gattex (Teduglutide)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The main aim of the study is to assess how well teduglutide works over 24 weeks in Chinese adult participants with short bowel syndrome (SBS) who need parenteral support and to see how much it can reduce the amount of parenteral support and understand how the body absorbs, processes, and gets rid of teduglutide. Participants will receive a daily injection of teduglutide under the skin for 24 weeks...
Summary: The main aims of this study are to check for side effects from treatment with teduglutide (Revestive) and how well teduglutide controls symptoms of short bowel syndrome. The study sponsor will not be involved in how the participants are treated but will provide instructions on how the clinics will record what happens during the study. During the study, participants with short bowel syndrome will r...
Summary: The main aim of this study is to document the level of knowledge and assess attitudes and behaviors of both participants and physicians regarding the risks and safe use of GATTEX. The survey will be done via internet, telephone, or paper and both physicians and participants will be able to choose the method that is preferred. No study medicines will be provided to participants in this study.
Related Latest Advances
Brand Information
- Acceleration of Neoplastic Growth
- Intestinal Obstruction
- Biliary and Pancreatic Disease
- Fluid Imbalance and Fluid Overload

- ask your healthcare provider or nurse to help you,
- ask someone who has been trained by a healthcare provider or nurse to give your injections.
- a healthcare provider or nurse,
- a parent or adult caregiver who has been trained by a healthcare provider or nurse to give injections of GATTEX to pediatric patients.
- Use of the GATTEX 5 mg kit is not recommended in pediatric patients weighing less than 22 pounds (10 kg).
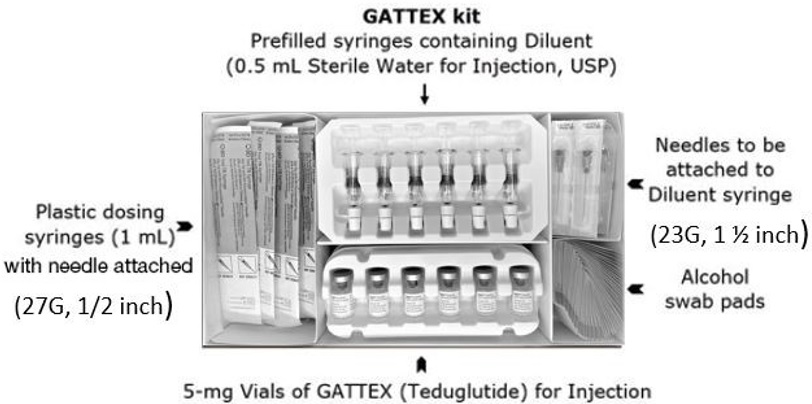
- Before you start, check the "Use By" date on your GATTEX kit. Make sure that the "Use By" date has not passed. Do not use anything in the GATTEX kit after the "Use By" date on the kit.
- Give GATTEX within 3 hours after you mix the powder with the Diluent (Sterile Water for Injection).
- Use the syringes and needles provided in the GATTEX kit.
- Do not use a GATTEX vial more than 1 time, even if there is medicine left in the vial.
- Throw away (dispose of) any unused GATTEX after you give your injection.
- Safely throw away GATTEX vials after use.
- Do not re-use syringes or needles. See " for information about how to safely throw away needles and syringes.
- To help avoid needle-stick injuries,
- Choose a well-lit, clean, flat work surface.
- Wash your hands with soap and water.
- Put the Diluent syringe
- Hold the Diluent syringe by the barrel. Snap off the white cap (bend the cap sideways until the cap comes off). Only the top portion of the white cap should be snapped off. The lower portion of the cap will remain in place
- Remove the 23G, 1½ inch needle from the package. Use the fold in the package to peel back the plastic cover
- Push the open end of the needle onto the end of the Diluent syringe
- When the needle is tightly in place, put the Diluent syringe and needle on your work surface.
- Remove the green cap from the GATTEX vial. Throw away the green cap.
- Find the gray rubber seal on top of the GATTEX vial
- Use an alcohol swab pad to clean the gray rubber seal
- Do not touch the gray rubber seal after you clean it.
- Pick up the Diluent syringe with the needle attached.
- Remove the plastic cap that covers the needle
- Hold the GATTEX vial between your thumb and index (pointer) finger
- Push the needle down through the center of the gray rubber seal.
- Slowly push down on the plunger of the Diluent syringe. Empty all the Diluent into the GATTEX vial.
- Leave the needle and Diluent syringe in place.
- Gently tap the barrel of the Diluent syringe with a finger
- Make sure all the Diluent has gone into the GATTEX vial.
- Remove the Diluent syringe and needle from the GATTEX vial. Let the vial sit for about 30 seconds.
- Do not put the needle cap back on the needle.
- Throw away (dispose of) the Diluent syringe and needle in your sharps disposal container.
- After 30 seconds, place the GATTEX vial between the palms of your hands. Gently roll the vial for about 15 seconds
- Do not shake the GATTEX vial.
- Do not touch the gray rubber seal. If you do, clean it again with a new alcohol pad.
- Let the GATTEX vial stand on your work surface for about 2 minutes.
- After 2 minutes, look at the vial of GATTEX. The liquid in the vial should be clear and colorless to pale yellow, and should not have any particles in it.
- If there is any powder in the GATTEX vial that did not dissolve, gently roll the vial between your hands for 15 seconds more.
- Do not shake the GATTEX vial.
- Check the GATTEX vial again for anything that did not dissolve.
- Do not use the GATTEX vial if there is anything in it that did not dissolve. Start from the beginning of this Instructions for Use to prepare a new vial. Use a new GATTEX vial, new Diluent syringe, and a new needle.
- Remove the plastic dosing syringe from the package. Use the fold in the package to peel back the plastic cover
- Remove the needle cap from the plastic dosing syringe (
- Throw the needle cap away. Do not touch the needle or allow it to touch anything.
- Carefully pull back on the plunger to the line that matches the dose prescribed by your healthcare provider.
- Use 1 hand to hold the GATTEX vial steady. Use your other hand to insert the needle straight down into the middle of the gray rubber seal on the GATTEX vial
- Gently push down the plunger until all of the air has gone from the plastic dosing syringe into the GATTEX vial.
- Turn the GATTEX vial and plastic dosing syringe upside down
- Hold the GATTEX vial with 1 hand.
- Slowly pull back the plunger of the plastic dosing syringe with your other hand.
- Fill the plastic dosing syringe until the black tip of the plunger lines up with the mark that matches your prescribed dose
- Keep the plastic dosing syringe and needle in the GATTEX vial.
- You may see some bubbles inside the GATTEX vial when the plastic dosing syringe is filled. This is normal. With the needle still in the vial, gently tap the side of the plastic dosing syringe with a finger to make any air bubbles rise to the top
- Slowly push the plunger up until all air bubbles are out of the plastic dosing syringe. Make sure the tip of the needle is in the fluid. Slowly pull back the plunger to draw up the right dose of GATTEX into the plastic dosing syringe.
- Remove the plastic dosing syringe and needle from the GATTEX vial
- Choose an injection site on the stomach area (abdomen), thighs, or upper arms.
- Choose a different site to give the injection each day. Do not inject into areas where the skin is tender, bruised, red, or hard.
- Clean the skin where you plan to give the injection with a new alcohol swab pad. Do not touch this area again before giving the injection.
- Use 1 hand to gently pinch up a fold of skin around the injection site
- Use your other hand to hold the plastic dosing syringe. Insert the full length of the needle into the skin at a 45-degree angle with a quick, "dart-like" motion
- Let go of the skin. Hold the syringe barrel with 1 hand while you slowly push down the plunger until the plastic dosing syringe is empty
- When the plastic dosing syringe is empty, quickly pull the needle out of your skin. There may be a little bleeding at the injection site. Apply an adhesive bandage to the injection site if needed.
- Do not re-use a syringe or needle.
- To help avoid needle-stick injuries, do not recap a needle.
- Put your needles and syringes in an FDA-cleared sharps disposal container right away after use.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
- Do not dispose of your sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your sharps disposal container.
- Throw away the GATTEX vial into the container where you put the syringes and needles.
- If you have any questions, talk to your healthcare provider or pharmacist.
- Store GATTEX powder at room temperature up to 77°F (25°C).
- Do not freeze GATTEX.
- Use the GATTEX powder by the expiration date on the "Use By" sticker on the kit.
- Use GATTEX within 3 hours after mixing it.
- Throw away any unused GATTEX that has been mixed, even if there is medicine left in the vial.
- Do not store any GATTEX you have mixed.
- Thirty single-dose vials of Gattex
- Package Insert
- Medication Guide
- For dispensing, transfer the product with vial trays

Apply Use By dating
sticker here

Apply Use By dating
sticker here




