Cardene
What is Cardene (Nicardipine)?
Approved To Treat
Related Clinical Trials
Summary: To investigate whether patients with cerebral vasospasm associated with aneurysmal subarachnoid hemorrhage have a better prognosis with intrathecal nicardipine injection via extraventricular drainage or lumbar drainage.
Summary: The overall objective of this double blinded, randomized controlled trial (RCT) is to compare specific outcomes of three medications (Dexmedetomidine, Nicardipine, and Labetalol) which are routinely used to lower blood pressure used during general anesthesia for orthognathic (jaw) surgery. The outcome measures for the study will be surgical field visibility, estimated blood loss, hemodynamic param...
Summary: The study investigators are interested in learning more about how drugs, that are given to children by their health care provider, act in the bodies of children and young adults in hopes to find the most safe and effective dose for children. The primary objective of this study is to evaluate the PK of understudied drugs currently being administered to children per SOC as prescribed by their treati...
Related Latest Advances
Brand Information
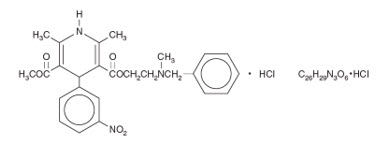

Nicardipine Hydrochloride
in 0.86% Sodium Chloride Injection
Rx only
Sterile, Nonpyrogenic
found, discard bag as sterility may be impaired. Do not use unless solution
is clear. Do not add supplemental medication. Must not be used in series
connections.


in 0.86 Sodium Chloride Injection
in 0.86 Sodium Chloride Injection
in 0.86 Sodium Chloride Injection
in 0.86 Sodium Chloride Injection
as sterility may be impaired. Do not use unless solution is clear. Do not add
supplemental medication. Must not be used in series connections.
Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
PROTECT FROM LIGHT, STORE IN CARTON UNTIL READY TO USE.
in 0.86 Sodium Chloride Injection
Nicardipine Hydrochloride
in 0.86 Sodium Chloride Injection


in 0.83% Sodium Chloride Injection
Rx only
Sterile, Nonpyrogenic
found, discard bag as sterility may be impaired. Do not use unless solution
is clear. Do not add supplemental medication. Must not be used in series
connections.
to USP Controlled Room Temperature. Protect from freezing. Avoid excessive
heat. PROTECT FROM LIGHT, STORE IN CARTON UNTIL READY TO USE.


in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
as sterility may be impaired. Do not use unless solution is clear. Do not add
supplemental medication. Must not be used in series connections.
Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
PROTECT FROM LIGHT, STORE IN CARTON UNTIL READY TO USE.
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
in 0.83% Sodium Chloride Injection
