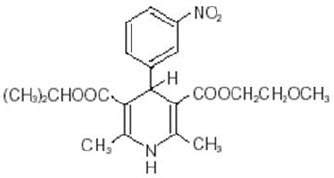
Nymalize
What is Nymalize (NiMODipine)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Aneurysmal subarachnoid hemorrhage (SAH) is a life-threatening neurological illness: it is bleeding in the brain after a bulging blood vessel (a brain aneurysm) ruptures. Although SAH accounts for only 5% of all strokes, it often happens in middle age and it puts a significant burden on many patients during their most productive years. Complications following SAH are common, and they can cause maj...
Summary: The role of perioperative IV administration of nimodipine, an L-type calcium channel antagonist which is capable of crossing the blood-brain barrier, on peri-operative opioid and anesthetics requirements, pain intensity, opioid-related side effects and early postoperative bowel mobility in patients undergoing surgical treatment for bowel cancer with open radical colectomy remains scarcely explored...
Summary: Electromagnetic fields (EMFs) generated by the use of 5G technology influence certain sleep characteristics, especially in individuals carrying a specific genetic variant of a protein in the brain that regulates the activity of nerve cells. This protein is a voltage-gated calcium channel called CaV1.2 and could be involved in the effects of 5G technology on sleep. The calcium channel CaV1.2 can be...
Related Latest Advances
Brand Information
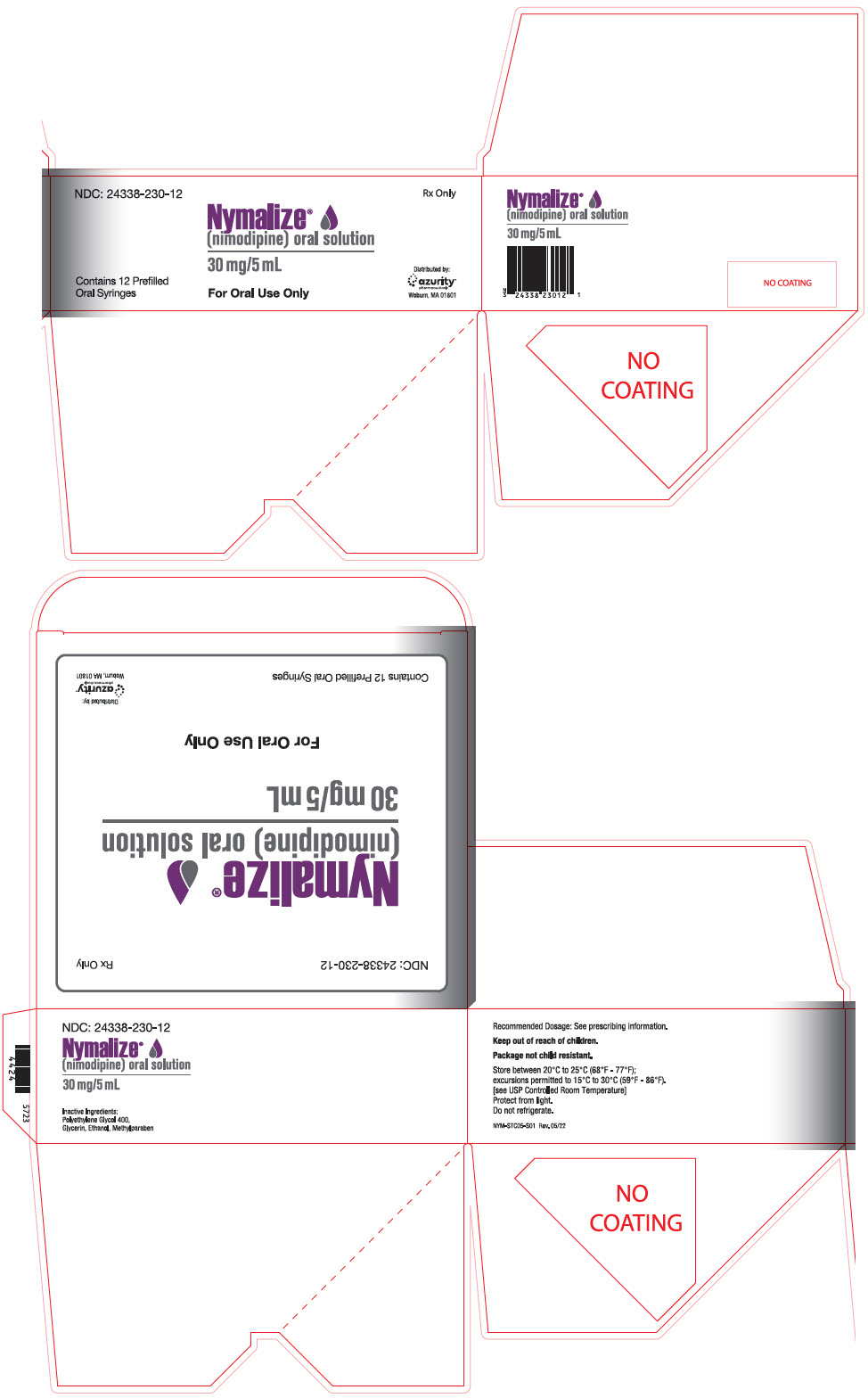
- 60 mg per 10 mL, pale yellow solution in unit-dose prefilled syringe
- 30 mg per 5 mL, pale yellow solution in unit-dose prefilled syringe
- 30 mg per 5 mL, pale yellow solution in unit-dose prefilled ENFit
- 60 mg per 10 mL (6 mg/mL), pale yellow solution in 8 oz bottle
- Hypotension
- NDC 24338-260-08: 8 oz. bottle (237 mL) 60 mg/10 mL (6 mg/mL)
- NDC 24338-260-12: Carton containing 12 individually wrapped 10 mL packages.
- NDC 24338-230-12: Carton containing 12 individually wrapped 5 mL packages.
- NDC 24338-230-30: Carton containing 12 blisters.