Brand Name
Targretin
Generic Name
Bexarotene
View Brand Information FDA approval date: December 29, 1999
Classification: Retinoid
Form: Capsule, Gel
What is Targretin (Bexarotene)?
Bexarotene capsules are indicated for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in patients who are refractory to at least one prior systemic therapy. Bexarotene is a retinoid indicated for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in patients who are refractory to at least one prior systemic therapy.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
A Phase Ib Trial Combining Bexarotene With Ultra-Low Dose Total Skin Electron Beam (Tseb) Radiotherapy For The Treatment Of Diffuse Cutaneous T-Cell Lymphomas
Summary: The researchers are doing this study to test the safety of combining bexarotene with TSEB radiotherapy in people who have a common form of CTCL called mycosis fungoides (MF). Bexarotene is a form of vitamin A that activates proteins called retinoid X receptors, which may stop the growth of cancer cells and kill them. TSEB radiotherapy is a type of radiation therapy that treats the entire surface o...
Related Latest Advances
Brand Information
Targretin (bexarotene)
WARNING: BIRTH DEFECTS
TARGRETIN is a member of the retinoid class of drugs that is associated with birth defects in humans. Bexarotene also caused birth defects when administered orally to pregnant rats. TARGRETIN must not be administered to a pregnant woman. (
1INDICATIONS AND USAGE
TARGRETIN
2DOSAGE AND ADMINISTRATION
The recommended initial dose of TARGRETIN is 300 mg/m
Dose Modification Guidelines: The 300 mg/m
Duration of Therapy: In clinical trials in CTCL, TARGRETIN was administered for up to 97 weeks.
TARGRETIN should be continued as long as the patient is deriving benefit.
3DOSAGE FORMS AND STRENGTHS
Capsules: 75 mg, off-white, oblong soft gelatin capsules, imprinted with black ink “Targretin”.
4ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the prescribing information:
- Hyperlipidemia [
- Pancreatitis [
- Hepatotoxicity, Cholestasis, and Hepatic Failure [
- Hypothyroidism [
- Neutropenia [
- Cataracts [
- Vitamin A Supplementation Hazard [
- Hypoglycemia Risk in Patients with Diabetes Mellitus [
- Photosensitivity [
- Laboratory Tests [
- Drug/Laboratory Test Interactions [
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of TARGRETIN has been evaluated in two clinical trials of 152 patients with CTCL who received TARGRETIN for up to 97 weeks and in 352 patients in other trials. The mean duration of therapy for the 152 patients with CTCL was 166 days. The most common adverse events reported with an incidence of at least 10% in patients with CTCL treated at an initial dose of 300 mg/m
Adverse reactions leading to TARGRETIN dose reduction or discontinuation in at least two patients were hyperlipemia, neutropenia/leukopenia, diarrhea, fatigue/lethargy, hypothyroidism, headache, liver function test abnormalities, rash, pancreatitis, nausea, anemia, allergic reaction, muscle spasm, pneumonia, and confusion.
The NCI Grade 3 and NCI Grade 4 adverse reactions reported in two or more patients with CTCL treated at an initial dose of 300 mg/m
In patients with CTCL receiving an initial dose of 300 mg/m
In addition to the 152 patients enrolled in the two CTCL trials, 352 patients received TARGRETIN as monotherapy for various advanced malignancies at doses from 5 mg/m
In the 504 patients (CTCL and non-CTCL) who received TARGRETIN as monotherapy, drug-related serious adverse reactions that were fatal, in one patient each, were acute pancreatitis, subdural hematoma, and liver failure.
In the patients with CTCL receiving an initial dose of 300 mg/m
Body as a Whole: chills, cellulitis, chest pain, breast pain, sepsis, and monilia infection.
Cardiovascular: hemorrhage, hypertension, angina pectoris, right heart failure, syncope, and tachycardia.
Digestive: constipation, dry mouth, flatulence, colitis, dyspepsia, cheilitis, gastroenteritis, gingivitis, liver failure, and melena.
Hemic and Lymphatic: eosinophilia, thrombocythemia, coagulation time increased, lymphocytosis, and thrombocytopenia.
Metabolic and Nutritional: LDH increased, creatinine increased, hypoproteinemia, hyperglycemia, weight decreased, weight increased, and amylase increased.
Musculoskeletal: arthralgia, myalgia, bone pain, myasthenia, and arthrosis.
Nervous: depression, agitation, ataxia, cerebrovascular accident, confusion, dizziness, hyperesthesia, hypesthesia, and neuropathy.
Respiratory: pharyngitis, rhinitis, dyspnea, pleural effusion, bronchitis, cough increased, lung edema, hemoptysis, and hypoxia.
Skin and Appendages: skin ulcer, acne, alopecia, skin nodule, macular papular rash, pustular rash, serous drainage, and vesicular bullous rash.
Special Senses: dry eyes, conjunctivitis, ear pain, blepharitis, corneal lesion, keratitis, otitis externa, and visual field defect.
Urogenital: albuminuria, hematuria, urinary incontinence, urinary tract infection, urinary urgency, dysuria, and kidney function abnormal.
Table 3: Incidence of Moderately Severe and Severe Adverse Events
Reported in at Least Two Patients (CTCL Trials)
Table 4: Treatment-Emergent Abnormal Laboratory Values in CTCL Trials
The safety profile from the one post-approval trial with 59 subjects was generally comparable to that of the pivotal trials with the exception of serious adverse events hypertriglyceridemia, neutropenia and bone marrow failure which were observed more frequently in the TARGRETIN 300 mg/m
Severe hypertriglyceridemia (≥800 mg/dL) was not seen in any subject in the lower dosage arm.
The most common AEs by preferred term in either the TARGRETIN 300 or 150 mg/m
Higher percentage of subjects in the TARGRETIN 300 mg/m
Of the SAEs of special interest, there were more events in the TARGRETIN 300 mg/m
5DRUG INTERACTIONS
Effect of Other Drugs on TARGRETIN
Gemfibrozil: Concomitant administration of TARGRETIN and gemfibrozil resulted in increases in plasma concentrations of bexarotene. Concomitant administration of gemfibrozil with TARGRETIN is not recommended.
Effect of TARGRETIN on Other Drugs
TARGRETIN may be an inducer for the CYP3A4 enzymes, and may reduce plasma concentrations of other substrates metabolized by CYP3A4. Drug products which may be affected include oral or other systemic hormonal contraceptives. Thus, if treatment with TARGRETIN is intended for a female with reproductive potential, it is strongly recommended that a non-hormonal contraception be considered. [
Laboratory Test Interference
CA125 assay values in patients with ovarian cancer may be increased by TARGRETIN therapy.
6OVERDOSAGE
Doses up to 1000 mg/m
7DESCRIPTION
TARGRETIN
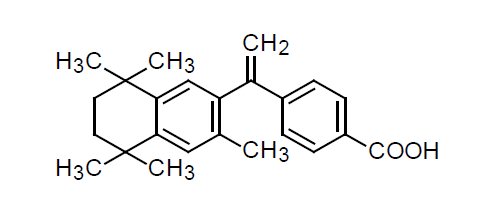
The chemical name of bexarotene is 4-[1-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl)ethenyl]benzoic acid, and the structural formula is as follows:
Bexarotene is an off-white to white powder with a molecular weight of 348.48 and a molecular formula of C
Each TARGRETIN capsule contains 75 mg of bexarotene for oral administration. It also contains the following inactive ingredients: butylated hydroxyanisole, NF, polyethylene glycol 400, NF, polysorbate 20, NF, and povidone, USP. The capsule shell contains gelatin, NF, sorbitol special glycerin blend, and titanium dioxide, USP.
8CLINICAL STUDIES
TARGRETIN was evaluated in two clinical trials in 152 patients with advanced and early stage cutaneous T-cell lymphoma (CTCL) in two multicenter, open-label, historically controlled clinical trials conducted in the U.S., Canada, Europe, and Australia.
The advanced disease patients had disease refractory to at least one prior systemic therapy (median of two, range 1 to 6 prior systemic therapies) and had been treated with a median of five (range 1 to 11) prior systemic, irradiation, and/or topical therapies. Early disease patients were intolerant to, had disease that was refractory to, or had reached a response plateau of 6 months on, at least two prior therapies. The patients entered had been treated with a median of 3.5 (range 2 to 12) therapies (systemic, irradiation, and/or topical).
The two clinical trials enrolled a total of 152 patients, 102 of whom had disease refractory to at least one prior systemic therapy, 90 with advanced disease and 12 with early disease. This is the patient population for whom TARGRETIN is indicated.
Patients were initially treated with a starting dose of 650 mg/m
Tumor response was assessed in both trials by observation of up to five baseline-defined index lesions using a Composite Assessment of Index Lesion Disease Severity (CA). This endpoint was based on a summation of the grades, for all index lesions, of erythema, scaling, plaque elevation, hypopigmentation or hyperpigmentation, and area of involvement. Also considered in response assessment was the presence or absence of cutaneous tumors and extracutaneous disease manifestations.
All tumor responses required confirmation over at least two assessments separated by at least 4 weeks. A partial response was defined as an improvement of at least 50% in the index lesions without worsening, or development of new cutaneous tumors or non-cutaneous manifestations. A complete clinical response required complete disappearance of all manifestations of disease, but did not require confirmation by biopsy.
At the initial dose of 300 mg/m
In one post-approval clinical trial with a total of 59 subjects (29 in 300 mg/m
The median duration of response in the TARGRETIN 300 mg/m
The median time to cutaneous tumor response (the time to cutaneous tumor response for a given subject is defined as the time interval from the first day of TARGRETIN treatment to the time of the first observation when the subject with subsequent confirmation of response meets criteria for CR, CCR or PR) in the TARGRETIN 300 mg/m
9HOW SUPPLIED/STORAGE AND HANDLING
TARGRETIN
Bottles of 100 capsules: NDC 0187-5526-75
Store at 2° to 25°C (36° to 77°F). Avoid exposing to high temperatures and humidity after the bottle is opened. Protect from light.
10PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform the patient or caregiver about the following:
Birth Defects
Advise patients that TARGRETIN is contraindicated in pregnancy
- Advise females of reproductive potential that they must avoid pregnancy while taking TARGRETIN and for at least 1 month following discontinuation of therapy.
- Advise females of reproductive potential of the importance of monthly pregnancy testing while taking TARGRETIN.
- Advise females of reproductive potential to use effective contraception for 1 month prior to the initiation of therapy, during therapy, and for at least 1 month following discontinuation of therapy and that two reliable forms of contraception should be used simultaneously, one of which should be non-hormonal.
- Advise females of reproductive potential that TARGRETIN therapy should be initiated on the second or third day of a normal menstrual period.
- Instruct patient to immediately stop taking TARGRETIN if she becomes pregnant while taking this drug.
- Advise male patients with sexual partners who are pregnant, possibly pregnant, or who could become pregnant that they must use condoms during sexual intercourse while taking TARGRETIN and for at least 1 month after the last dose of the drug.
Pancreatitis
Advise patients of the risk of developing pancreatitis, which may be accompanied by nausea, vomiting, and abdominal or back pain and to immediately contact their healthcare provider if these symptoms occur
Hepatotoxicity
Inform patients of the possibility of developing liver function abnormalities and serious hepatic toxicity. Advise patients to immediately contact their healthcare provider if signs of liver failure occur, including jaundice, anorexia, bleeding, or bruising
Neutropenia
Advise patients of the possibility of developing neutropenia and to immediately contact their healthcare provider should they develop a fever, particularly in association with any suggestion of infection
Cataracts
Advise patients of the possibility of developing new or worsening cataracts and to inform their healthcare provider about any changes in their vision during treatment with TARGRETIN
Vitamin A Supplementation Hazard
Advise patients to limit vitamin A intake to ≤15,000 IU/day to avoid potential additive toxic effects.
Hypoglycemia and Diabetes Mellitus
Advise patients of the possibility of developing hypoglycemia when using insulin, agents enhancing insulin secretion, or insulin sensitizers while on TARGRETIN therapy. Instruct patients on these medications to check their blood sugar frequently and to notify their physicians of any changes in blood sugar level
Photosensitivity
Advise patients of potential increased skin sensitivity to sunlight while taking TARGRETIN and to minimize exposure to sunlight and artificial ultraviolet light
Laboratory Tests
Advise patients of laboratory testing which will occur during therapy to monitor lipids, liver function, thyroid function, and white blood cell counts
Administration Instructions
Advise patients to take TARGRETIN with a meal
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Catalent Pharma Solutions LLC
St. Petersburg, FL 33716 USA
TARGRETIN is a trademark of Bausch Health Companies Inc. or its affiliates.
Any other product/brand names are trademarks of their respective owners.
© 2020 Bausch Health Companies Inc. or its affiliates
9436601
11PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – TARGRETIN – 75 mg Carton
NDC 0187-5526-75
Targretin
(bexarotene) capsules
(bexarotene) capsules
75 mg
100 Capsules
Rx Only
Bausch Health Companies