Generic Name
MethylTESTOSTERone
Brand Names
Estratest, Methitest
FDA approval date: October 17, 1974
Classification: Androgen
Form: Tablet, Capsule
What is Estratest (MethylTESTOSTERone)?
ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE FULL STRENGTH and ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE HALF STRENGTH are indicated in the treatment of: Moderate to severe vasomotor symptoms associated with the menopause in those patients not improved by estrogens alone. ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE FULL STRENGTH and ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE HALF STRENGTH HAVE NOT BEEN SHOWN TO BE EFFECTIVE FOR ANY PURPOSE DURING PREGNANCY AND ITS USE MAY CAUSE SEVERE HARM TO THE FETUS .
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
Evaluating Novel Pediatric Pulse Oximeters for Outpatient Child Pneumonia Care in Sub-Saharan Africa
Evaluating Novel Pediatric Pulse Oximeters for Outpatient Child Pneumonia Care in Sub-Saharan Africa
Summary: The primary objective of this clinical trial is to evaluate the performance of three pulse oximeters during outpatient care within Cape Town, South Africa. This objective will be achieved through generating evidence on how, why, for whom, to what extent and at what cost can paediatric pulse oximetry devices improve the management of hypoxemic children. This will be done with two inter-linked studi...
Related Latest Advances
Brand Information
ESTRATEST H.S. (ESTERIFIED ESTROGENS, METHYLTESTOSTERONE)
WARNINGS
1. ESTROGENS HAVE BEEN REPORTED TO INCREASE THE RISK OF ENDOMETRIAL CARCINOMA
Three independent case control studies have reported an increased risk of endometrial cancer in postmenopausal women exposed to exogenous estrogens for prolonged periods.
The three case control studies reported that the risk of endometrial cancer in estrogen users was about 4.5 to 13.9 times greater than in nonusers. The risk appears to depend on both duration of treatment1 and on estrogen dose.
Close clinical surveillance of all women taking estrogens is important. In all cases of undiagnosed persistent or recurring abnormal vaginal bleeding, adequate diagnostic measures should be undertaken to rule out malignancy.
There is no evidence at present that "natural" estrogens are more or less hazardous than "synthetic" estrogens at equiestrogenic doses.
2. ESTROGENS SHOULD NOT BE USED DURING PREGNANCY
The use of female sex hormones, both estrogens and progestogens, during early pregnancy may seriously damage the offspring. It has been shown that females exposed in utero to diethylstilbestrol, a non-steroidal estrogen, have an increased risk of developing in later life a form of vaginal or cervical cancer that is ordinarily extremely rare.
Several reports suggest an association between intrauterine exposure to female sex hormones and congenital anomalies, including congenital heart defects and limb reduction defects.
In the past, female sex hormones have been used during pregnancy in an attempt to treat threatened or habitual abortion. There is considerable evidence that estrogens are ineffective for these indications, and there is no evidence from well controlled studies that progesterones are effective for these uses.
IF ESTRATEST H.S. AND ESTRATEST F.S. is used during pregnancy, or if the patient becomes pregnant while taking this drug, she should be apprised of the potential risks to the fetus, and the advisability of pregnancy continuation.
1DESCRIPTION
ESTRATEST F.S.: Each light yellow, capsule-shaped, film-coated oral tablet contains: 1.25 mg of Esterified Estrogens, USP and 2.5 mg of Methyltestosterone, USP.
ESTRATEST H.S.: Each light pink, capsule-shaped, film-coated oral tablet contains: 0.625 mg of Esterified Estrogens, USP and 1.25 mg of Methyltestosterone, USP.
Esterified Estrogens
Esterified Estrogens, USP is a mixture of the sodium salts of the sulfate esters of the estrogenic substances, principally estrone, that are of the type excreted by pregnant mares. Esterified Estrogens contain not less than 75.0 percent and not more than 85.0 percent of sodium estrone sulfate, and not less than 6.0 percent and not more than 15.0 percent of sodium equilin sulfate, in such proportion that the total of these two components is not less than 90.0 percent.
Category: Estrogens
Methyltestosterone
Methyltestosterone is an androgen. Androgens are derivatives of cyclopentano-perhydrophenanthrene. Endogenous androgens are C-19 steroids with a side chain at C-17, and with two angular methyl groups. Testosterone is the primary endogenous androgen. Fluoxymesterone and methyltestosterone are synthetic derivatives of testosterone.
Methyltestosterone is a white to light yellow crystalline substance that is virtually insoluble in water but soluble in organic solvents. It is stable in air but decomposes in light.
Methyltestosterone structural formula:
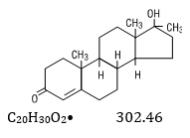
Androst-4-en-3-one, 17-hydroxy-17-methyl-, (17B)-.Category: Androgen.
ESTRATEST H.S. AND ESTRATEST F.S. TABLETS contain the following inactive ingredients: lactose, magnesium stearate, microcrystalline cellulose, titanium dioxide, and other minor ingredients.
ESTRATEST F.S. TEST also contain: Opadry Clear, FD&C Yellow #6, FD&C Yellow #5
ESTRATEST H.S. TABLETS also contain: Opadry Clear, D&C Red #30
2CLINICAL PHARMACOLOGY
Estrogens
Estrogens are important in the development and maintenance of the female reproductive system and secondary sex characteristics. They promote growth and development of the vagina, uterus, and fallopian tubes, and enlargement of the breasts. Indirectly, they contribute to the shaping of the skeleton, maintenance of tone and elasticity of urogenital structures, changes in the epiphyses of the long bones that allow for the pubertal growth spurt and its termination, growth of axillary and pubic hair, and pigmentation of the nipples and genitals. Decline of estrogenic activity at the end of the menstrual cycle can bring on menstruation, although the cessation of progesterone secretion is the most important factor in the mature ovulatory cycle. However, in the preovulatory or nonovulatory cycle, estrogen is the primary determinant in the onset of menstruation. Estrogens also affect the release of pituitary gonadotropins. The pharmacologic effects of esterified estrogens are similar to those of endogenous estrogens. They are soluble in water and are well absorbed from the gastrointestinal tract.
In responsive tissues (female genital organs, breasts, hypothalamus, pituitary) estrogens enter the cell and are transported into the nucleus. As a result of estrogen action, specific RNA and protein synthesis occurs.
Estrogen Pharmacokinetics
Metabolism and inactivation occur primarily in the liver. Some estrogens are excreted into the bile; however they are reabsorbed from the intestine and returned to the liver through the portal venous system. Water soluble esterified estrogens are strongly acidic and are ionized in body fluids, which favor excretion through the kidneys since tubular reabsorption is minimal.
Androgens
Endogenous androgens are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as beard, pubic, chest and axillary hair, laryngeal enlargement, vocal cord thickening, alterations in body musculature, and fat distribution. Drugs in this class also cause retention of nitrogen, sodium, potassium, phosphorus, and decreased urinary excretion of calcium. Androgens have been reported to increase protein anabolism and decrease protein catabolism. Nitrogen balance is improved only when there is sufficient intake of calories and protein. Androgens are responsible for the growth spurt of adolescence and for the eventual termination of linear growth centers. In children, exogenous androgens accelerate linear growth rates, but may cause a disproportionate advancement in bone maturation. Use over long periods may result in fusion of the epiphyseal growth centers and termination of growth process. Androgens have been reported to stimulate the production of red blood cells by enhancing the production of erythropoietic stimulating factor.
Androgen Pharmacokinetics
Testosterone given orally is metabolized by the gut and 44 percent is cleared by the liver in the first pass. Oral doses as high as 400 mg per day are needed to achieve clinically effective blood levels for full replacement therapy. The synthetic androgens (methyltestosterone and fluoxymesterone) are less extensively metabolized by the liver and have longer half-lives. They are more suitable than testosterone for oral administration.
Testosterone in plasma is 98 percent bound to a specific testosterone estradiol binding globulin, and about 2 percent is free. Generally, the amount of this sex-hormone binding globulin in the plasma will determine the distribution of testosterone between free and bound forms, and the free testosterone concentration will determine its half-life.
About 90 percent of a dose of testosterone is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6 percent of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver. Testosterone is metabolized to various 17-keto steroids through two different pathways. There are considerable variations of the half-life of testosterone as reported in the literature, ranging from 10 to 100 minutes.
In many tissues the activity of testosterone appears to depend on reduction to dihydrotestosterone, which binds to cytosol receptor proteins. The steroid-receptor complex is transported to the nucleus where it initiates transcription events and cellular changes related to androgen action.
3INDICATIONS AND USAGE
ESTRATEST H.S. AND ESTRATEST F.S. are indicated in the treatment of: Moderate to severe
ESTRATEST H.S. AND ESTRATEST F.S. HAVE NOT BEEN SHOWN TO BE EFFECTIVE FOR ANY PURPOSE DURING PREGNANCY AND ITS USE MAY CAUSE SEVERE HARM TO THE FETUS (SEE
4CONTRAINDICATIONS
Estrogens should not be used in women with any of the following conditions:
1. Known or suspected cancer of the breast except in appropriately selected patients being treated for metastatic disease.
2. Known or suspected estrogen-dependent neoplasia.
3. Known or suspected pregnancy (See
4. Undiagnosed abnormal genital bleeding.
5. Active thrombophlebitis or thromboembolic disorders.
6. A past history of thrombophlebitis, thrombosis, or thromboembolic disorders associated with previous estrogen use (except when in treatment of breast malignancy).
Methyltestosterone should not be used in:
1. The presence of severe liver damage.
2. Pregnancy and in breast-feeding mothers because of the possibility of masculinization of the female fetus or breast-fed infant.
5WARNINGS
Associated with Estrogens
- Induction of malignant neoplasms. Long term continuous administration of natural and synthetic estrogens in certain animal species increases this frequency of carcinomas of the breast, cervix, vagina, and liver. There is now evidence that estrogens increase the risk of carcinoma of the endometrium in humans (See Boxed Warning).
At the present time there is no satisfactory evidence that estrogens given to postmenopausal women increase the risk of cancer of the breast,
2.
3.
a. Thromboembolic disease. It is now well established that users of oral contraceptives have an increased risk of various thromboembolic and thrombotic vascular diseases, such as thrombophlebitis, pulmonary embolism, stroke, and myocardial infarction.
While an increased rate of thromboembolic and thrombotic disease in postmenopausal users of estrogens has not been found,
Large doses of estrogen (5 mg esterified estrogens per day), comparable to those used to treat cancer of the prostate and breast, have been shown in a large prospective clinical trial in men
b. Hepatic adenoma. Benign hepatic adenomas appear to be associated with the use of oral contraceptives.
c. Elevated blood pressure. Increased blood pressure is not uncommon in women using oral contraceptives. There is now a report that this may occur with use of estrogens in the menopause
d. Glucose tolerance. A worsening of glucose tolerance has been observed in a significant percentage of patients of estrogen-containing oral contraceptives. For this reason, diabetic patients should be carefully observed while receiving estrogens.
4.
Associated with Methyltestosterone
In patients with breast cancer, androgen therapy may cause hypercalcemia by stimulating osteolysis. In this case the drug should be discontinued.
Prolonged use of high doses of androgens has been associated with the development of peliosis hepatis and hepatic neoplasms including hepatocellular carcinoma. (See
Peliosis hepatis can be a life-threatening or fatal complication.
Cholestatic hepatitis and jaundice occur with 17-alpha-alkyl androgens at a relatively low dose. If cholestatic hepatitis with jaundice appears or if liver function tests become abnormal, the androgen should be discontinued and the etiology should be determined. Drug-induced jaundice is reversible when the medication is discontinued.
Edema with or without heart failure may be a serious complication in patients with preexisting cardiac, renal, or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required.
6PRECAUTIONS
Associated with Estrogens
A. General Precautions
1. A complete medical and family history should be taken prior to the initiation of any estrogen therapy. The pretreatment and periodic physical examinations should include special reference to blood pressure, breasts, abdomen, and pelvic organs, and should include a Papanicolaou smear. As a general rule, estrogen should not be prescribed for longer than one year without another physical examination being performed.
2. Fluid retention– Because estrogens may cause some degree of fluid retention, conditions which might be influenced by this factor such as asthma, epilepsy, migraine, and cardiac or renal dysfunction, require careful observation.
3. Certain patients may develop undesirable manifestations of excessive estrogenic stimulation, such as abnormal or excessive uterine bleeding, mastodynia, etc.
4. Oral contraceptives appear to be associated with an increased incidence of mental depression.
5. Preexisting uterine leiomyomata may increase in size during estrogen use.
6. The pathologist should be advised of estrogen therapy when relevant specimens are submitted.
7. Patients with a past history of jaundice during pregnancy have an increased risk of recurrence of jaundice while receiving estrogen-containing oral contraceptive therapy. If jaundice develops in any patient receiving estrogen, the medication should be discontinued while the cause is investigated.
8. Estrogens may be poorly metabolized in patients with impaired liver function and they should be administered with caution in such patients.
9. Because estrogens influence the metabolism of calcium and phosphorus, they should be used with caution in patients with metabolic bone diseases that are associated with hypercalcemia or in patients with renal insufficiency.
10. Because of the effects of estrogens on epiphyseal closure, they should be used judiciously in young patients in whom bone growth is not complete.
11. Certain endocrine and liver function tests may be affected by estrogen-containing oral contraceptives. The following similar changes may be expected with larger doses of estrogen:
B. Information for the Patient
See text of Patient Package Insert which appears after the REFERENCES.
C. Pregnancy Category X
See
D. Nursing Mothers
As a general principle, the administration of any drug to nursing mothers should be done only when clearly necessary since many drugs are excreted in human milk.
Associated with Methyltestosterone
6.1H. Nursing Mothers
It is not known whether androgens are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from androgens, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
7ADVERSE REACTIONS
Associated with Estrogens
(See
- Genitourinary system.
Breakthrough bleeding, spotting, change in menstrual flow.
Dysmenorrhea.
Premenstrual-like syndrome.
Amenorrhea during and after treatment.
Increase in size of uterine fibromyomata.
Vaginal candidiasis.
Change in cervical erosion and in degree of cervical secretion.
Cystitis-like syndrome. - Breasts.
Tenderness, enlargement, secretion. - Gastrointestinal.
Nausea, vomiting.
Abdominal cramps, bloating.
Cholestatic jaundice. - Skin.
Chloasma or melasma which may persist when drug is discontinued.
Erythema multiforme.
Erythema nodosum.
Hemorrhagic eruption.
Loss of scalp hair.
Hirsutism. - Eyes.
Steepening of corneal curvature.
Intolerance to contact lenses. - CNS.
Headache, migraine, dizziness.
Mental depression.
Chorea. - Miscellaneous.
Increase or decrease in weight.
Reduced carbohydrate tolerance.
Aggravation of porphyria.
Edema.
Changes in libido.
Associated with Methyltestosterone
A. Endocrine and Urogenital.
1.
2.
3.
4.
5.
6.
7.
8.
8OVERDOSAGE
Numerous reports of ingestion of large doses of estrogen-containing oral contraceptives by young children indicate that serious ill effects do not occur. Overdosage of estrogen may cause nausea, and withdrawal bleeding may occur in females.
There have been no reports of acute overdosage with the androgens.
9DOSAGE AND ADMINISTRATION
1.
For treatment of moderate to severe vasomotor symptoms associated with the menopause in patients not improved by estrogen alone.
The lowest dose that will control symptoms should be chosen and medication should be discontinued as promptly as possible.
Administration should be cyclic (e.g., three weeks on and one week off). Attempts to discontinue or taper medication should be made at three to six month intervals.
Usual Dosage Range
1 tablet of ESTRATEST F.S. or 1 to 2 tablets of ESTRATEST H.S. daily as recommended by the physician.
Treated patients with an intact uterus should be monitored closely for signs of endometrial cancer and appropriate diagnostic measures should be taken to rule out malignancy in the event of persistent or recurring abnormal vaginal bleeding.
10HOW SUPPLIED
ESTRATEST F.S. in bottles of 100.
ESTRATEST F.S. light yellow, capsule-shaped, film-coated, debossed "C010" on obverse and plain on the reverse. Contains: 1.25 mg of Esterified Estrogens, USP and 2.5 mg of Methyltestosterone, USP.
ESTRATEST H.S. in bottles of 100.
ESTRATEST H.S. light pink, capsule-shaped, film-coated, debossed "C020" on obverse and plain on the reverse. Contains: 0.625 mg of Esterified Estrogens, USP and 1.25 mg of Methyltestosterone, USP.
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature.]
Rx only
11REFERENCES
1. Ziel, H.K.
2. Smith, D.C.,
3. Mack, T.M.,
4. Weiss, N.S.
5. Herbst, A.L.
6. Greenwald, P.,
7. Lanier, A.,
8. Herbst, A.,
9. Herbst, A.,
10. Herbst, A.,
11. Stafl, A.,
12. Sherman, A.I.,
13. Gal, I.,
14. Levy, E.P.,
15. Nora, J.,
16. Janerich, D.T.,
17. Estrogens for Oral or Parenteral Use: Federal Register
18. Boston Collaborative Drug Surveillance Program: N. Engl. J. Med.
18a. Hoover, R.
19. Boston Collaborative Drug Surveillance Program: Lancet
20. Daniel, D.G.,
21. The Veterans Administration Cooperative Urological Research Group: J. Urol,
22. Bailar, J.C.: Lancet
23. Blackard, C.,
24. Royal College of General Practitioners: J.R. Coll, Gen. Pract.
25. Inman, W.H.W.,
26. Vessey, M.P.,
27. Sartwell, P.E.,
28. Collaborative Group for the Study of Stroke in Young Women: N. Engl. J. Med.
29. Collaborative Group for the Study of Stroke in Young Women: J.A.M.A
30. Mann, J.I.,
31. Mann, J.I.,
32. Inman, W.H.W.,
33. Stolley, P.D.,
34. Vessey, M.P.,
35. Greene, G.R.,
36. Rosenberg, L.,
37. Coronary Drug Project Research Group: J.A.M.A.
38. Baum, J.,
39. Mays, E.T.,
40. Edmondson, H.A.,
41. Pfeffer, R.I.,
Rx only
Distributed By:
Method Pharmaceuticals, LLC
Southlake, TX 76092
Rev. 05/24
12PATIENT INFORMATION
WHAT YOU SHOULD KNOW ABOUT ESTRATEST H.S. and ESTRATEST F.S.
WHAT YOU SHOULD KNOW ABOUT ESTROGENS
Estrogens are female hormones produced by the ovaries. The ovaries make several different kinds of estrogens. In addition, scientists have been able to make a variety of synthetic estrogens. As far as we know, all these estrogens have similar properties and therefore much the same usefulness, side effects, and risks. This leaflet is intended to help you understand what estrogens are used for, the risks involved in their use, and how to use them as safely as possible.
This leaflet includes the most important information about estrogens, but not all the information. If you want to know more, you can ask your doctor or pharmacist to let you read the package insert prepared for the doctor.
USES OF ESTROGEN
Estrogens are prescribed by doctors for a number of purposes, including:
- To provide estrogen during a period of adjustment when a woman’s ovaries no longer produce it, in order to prevent certain uncomfortable symptoms of estrogen deficiency. (All women normally stop producing estrogens, generally between the ages of 45 and 55; this is called the menopause).
- To prevent symptoms of estrogen deficiency when a woman’s ovaries have been removed surgically before the natural menopause.
- To prevent pregnancy: (Estrogens are given along with progestogen, another female hormone; these combinations are called oral contraceptives or birth control pills. Patient labeling is available to women taking oral contraceptives and they will not be discussed in this leaflet.)
- To treat certain cancers in women and men.
THERE IS NO PROPER USE OF ESTROGENS IN A PREGNANT WOMAN.
ESTROGENS IN THE MENOPAUSE
In the natural course of their lives, all women eventually experience a decrease in estrogen production. This usually occurs between ages 45 and 55 but may occur earlier or later. Sometimes the ovaries may need to be removed before natural menopause by an operation, producing a “surgical menopause.”
When the amount of estrogen in the blood begins to decrease, many women may develop typical symptoms: Feelings of warmth in the face, neck, and chest or sudden intense episodes of heat and sweating throughout the body (called “hot flashes” or “hot flushes”). These symptoms are sometimes very uncomfortable. A few women eventually develop changes in the vagina (called “atrophic vaginitis”) which cause discomfort, especially during and after intercourse.
Estrogens can be prescribed to treat these symptoms of the menopause. It is estimated that considerably more than half of all women undergoing the menopause have only mild symptoms or no symptoms at all and therefore do not need estrogens. Other women may need estrogens for a few months, while their bodies adjust to lower estrogen levels. Sometimes the need will be for periods longer than six months. In an attempt to avoid overstimulation of the uterus (womb), estrogens are usually given cyclically during each month of use, that is three weeks of pills followed by one week without pills.
Sometimes women experience nervous symptoms or depression during menopause. There is no evidence that estrogens are effective for such symptoms and they should not be used to treat them, although other treatment may be needed.
You may have heard that taking estrogens for long periods (years) after the menopause will keep your skin soft and supple and keep you feeling young. There is no evidence that this is so, however, and such long-term treatment carries important risks.
THE DANGERS OF ESTROGENS
1. Cancer of the uterus. If estrogens are used in the postmenopausal period for more than a year, there is an increased risk of endometrial cancer(cancer of the uterus). Women taking estrogens have roughly 5 to 10 times as great a chance of getting this cancer as women who take no estrogens. To put this another way, while a postmenopausal woman not taking estrogens has 1 chance in 1,000 each year of getting cancer of the uterus, a woman taking estrogens has 5 to 10 chances in 1,000 each year. For this reason it is important to take estrogens only when you really need them.
The risk of this cancer is greater the longer estrogens are used and also seems to be greater when larger doses are taken. For this reason, it is important to take the lowest dose of estrogen that will control symptoms and to take it only as long as it is needed. If estrogens are needed for longer periods of time, your doctor will want to reevaluate your need for estrogens at least every six months.
Women using estrogens should report any irregular vaginal bleeding to their doctors; such bleeding may be of no importance, but it can be an early warning of cancer of the uterus. If you have undiagnosed vaginal bleeding, you should not use estrogens until a diagnosis is made and you are certain there is no cancer of the uterus.
2. Other possible cancers. Estrogens can cause development of other tumors in animals, such as tumors of the breast, cervix, vagina, or liver, when given for a long time. At present there is no good evidence that women using estrogen in the menopause have an increased risk of such tumors, but there is no way yet to be sure they do not; and one study raises the possibility that use of estrogens in the menopause may increase the risk of breast cancer many years later. This is a further reason to use estrogens only when clearly needed. While you are taking estrogens, it is important that you go to your doctor at least once a year for a physical examination. Also, if members of your family have had breast cancer or if you have breast nodules or abnormal mammograms (breast x-rays), your doctor may wish to carry out more frequent examinations of your breasts.
3. Gallbladder disease. Women who use estrogens after menopause are more likely to develop gallbladder disease needing surgery as women who do not use estrogens. Birth control pills have a similar effect.
4. Abnormal blood clotting. Oral contraceptives increase the risk of blood clotting in various parts of the body.
The risk of this cancer is greater the longer estrogens are used and also seems to be greater when larger doses are taken. For this reason, it is important to take the lowest dose of estrogen that will control symptoms and to take it only as long as it is needed. If estrogens are needed for longer periods of time, your doctor will want to reevaluate your need for estrogens at least every six months.
Women using estrogens should report any irregular vaginal bleeding to their doctors; such bleeding may be of no importance, but it can be an early warning of cancer of the uterus. If you have undiagnosed vaginal bleeding, you should not use estrogens until a diagnosis is made and you are certain there is no cancer of the uterus.
2. Other possible cancers. Estrogens can cause development of other tumors in animals, such as tumors of the breast, cervix, vagina, or liver, when given for a long time. At present there is no good evidence that women using estrogen in the menopause have an increased risk of such tumors, but there is no way yet to be sure they do not; and one study raises the possibility that use of estrogens in the menopause may increase the risk of breast cancer many years later. This is a further reason to use estrogens only when clearly needed. While you are taking estrogens, it is important that you go to your doctor at least once a year for a physical examination. Also, if members of your family have had breast cancer or if you have breast nodules or abnormal mammograms (breast x-rays), your doctor may wish to carry out more frequent examinations of your breasts.
3. Gallbladder disease. Women who use estrogens after menopause are more likely to develop gallbladder disease needing surgery as women who do not use estrogens. Birth control pills have a similar effect.
4. Abnormal blood clotting. Oral contraceptives increase the risk of blood clotting in various parts of the body.
This can result in a stroke (if the clot is in the brain), a heart attack (clot in a blood vessel of the heart), or pulmonary embolus (a clot which forms in the legs or pelvis, then breaks off and travels to the lungs). Any of these can be fatal.
SPECIAL WARNING ABOUT PREGNANCY
You should not receive estrogen if you are pregnant. If this should occur, there is a greater than usual chance that the developing child will be born with a birth defect, although the possibility remains fairly small. A female child may have an increased risk of developing cancer of the vagina or cervix later in life (in the teens or twenties). Every possible effort should be made to avoid exposure to estrogens during pregnancy. If exposure occurs, see your doctor.
OTHER EFFECTS OF ESTROGENS
In addition to the serious known risks of estrogens described above, estrogens have the following side effects and potential risks:
1. Nausea and vomiting. The most common side effect of estrogen therapy is nausea. Vomiting is less common.
2. Effects on breasts. Estrogens may cause breast tenderness or enlargement and may cause the breasts to secrete a liquid. These effects are not dangerous.
3. Effects on the uterus. Estrogens may cause benign fibroid tumors of the uterus to get larger.
Some women will have menstrual bleeding when estrogens are stopped. But if the bleeding occurs on days you are still taking estrogens you should report this to your doctor.
4. Effects on liver. Women taking oral contraceptives develop on rare occasions a benign tumor of the liver which can rupture and bleed into the abdomen. So far, these tumors have not been reported in women using estrogens in the menopause, but you should report any swelling or unusual pain or tenderness in the abdomen to your doctor immediately. Women with a past history of jaundice (yellowing of the skin and white parts of the eyes), may get jaundice again during estrogen use. If this occurs, stop taking estrogens and see your doctor.
5. Other effects. Estrogens may cause excess fluid to be retained in the body. This may make some conditions worse, such as epilepsy, migraine, heart disease, or kidney disease.
2. Effects on breasts. Estrogens may cause breast tenderness or enlargement and may cause the breasts to secrete a liquid. These effects are not dangerous.
3. Effects on the uterus. Estrogens may cause benign fibroid tumors of the uterus to get larger.
Some women will have menstrual bleeding when estrogens are stopped. But if the bleeding occurs on days you are still taking estrogens you should report this to your doctor.
4. Effects on liver. Women taking oral contraceptives develop on rare occasions a benign tumor of the liver which can rupture and bleed into the abdomen. So far, these tumors have not been reported in women using estrogens in the menopause, but you should report any swelling or unusual pain or tenderness in the abdomen to your doctor immediately. Women with a past history of jaundice (yellowing of the skin and white parts of the eyes), may get jaundice again during estrogen use. If this occurs, stop taking estrogens and see your doctor.
5. Other effects. Estrogens may cause excess fluid to be retained in the body. This may make some conditions worse, such as epilepsy, migraine, heart disease, or kidney disease.
SUMMARY
Estrogens have important uses, but they have serious risks as well. You must decide, with your doctor, whether the risks are acceptable to you in view of the benefits of the treatment. Except where your doctor had prescribed estrogens for use in special cases of cancer of the breast or prostate, you should not use estrogens if you have cancer of the breast or uterus, are pregnant, have undiagnosed abnormal vaginal bleeding, or have had a stroke, heart attack or angina, or clotting in the legs or lungs in the past while you were taking estrogens.
You can use estrogens as safely as possible by understanding that your doctor will require regular physical examinations while you are taking them and will try to discontinue the drug as soon as possible and use the smallest dose possible. Be alert for signs of trouble including:
- Abnormal bleeding from the vagina.
- Pains in the calves or chest or sudden shortness of breath, or coughing blood (indicating possible clots in the legs, heart, or lungs).
- Severe headache, dizziness, faintness, or changes in vision (indicating possible developing clots in the brain or eye).
4. Breast lumps (you should ask your doctor how to examine your own breasts).
5. Jaundice (yellowing of the skin).
Based on his or her assessment of your medical needs, your doctor has prescribed this drug for you. Do not give the drug to anyone else.
HOW SUPPLIED
ESTRATEST H.S. a combination of Esterified Estrogens and Methyltestosterone.
Each capsule-shaped, light pink, film-coated tablet contains: 0.625 mg of Esterified Estrogens, USP and 1.25 mg of Methyltestosterone, USP.
ESTRATEST F.S. a combination of Esterified Estrogens and Methyltestosterone.
Each capsule-shaped, light yellow, film-coated tablet contains: 1.25 mg of Esterified Estrogens, USP and 2.5 mg of Methyltestosterone, USP.
All prescription substitutions using this product shall be pursuant to state statutes as applicable.
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature.]
Rx only
Manufactured By:
Southlake, TX 76092
13PACKAGE LABEL-PRINCIPAL DISPLAY PANEL
ESTRATEST F.S. - NDC 58657-404-01 - 100 Tablets Label

ESTRATEST H.S. - NDC 58657-403-01 - 100 Tablets Label

