Entyvio
What is Entyvio (Vedolizumab)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to estimate and compare the incidence of overall malignancy, serious infection, and opportunistic infections between new users of ustekinumab and new users of other biologic therapies among adult participants with Crohn's disease (CD) or ulcerative colitis (UC).
Summary: Ulcerative Colitis (UC) and Crohn's Disease (CD) are long-term conditions in the gut that can cause diarrhea, swelling (inflammation), bleeding from the anus, and belly pain. The main aim of this study is to check for how many participants with UC and CD signs and symptoms disappear after 3.5 months (14 weeks) of treatment with Vedolizumab (this is called remission). Participants will be treated w...
Summary: The main aim of the study is to observe adult participants in South Korea that are being treated with vedolizumab injected just under the skin (subcutaneous or SC) to treat ulcerative colitis (UC) or Crohn's disease (CD) who have had an in-adequate response with, lost response to, or had too many side effects in response to either conventional therapy or a Tumor Necrosis Factor-alpha (TNF-α) antag...
Related Latest Advances
Brand Information
- moderately to severely active ulcerative colitis (UC).
- moderately to severely active Crohn's disease (CD).
- Infusion-Related Reactions and Hypersensitivity Reactions
- Infections
- Progressive Multifocal Leukoencephalopathy
- Liver Injury
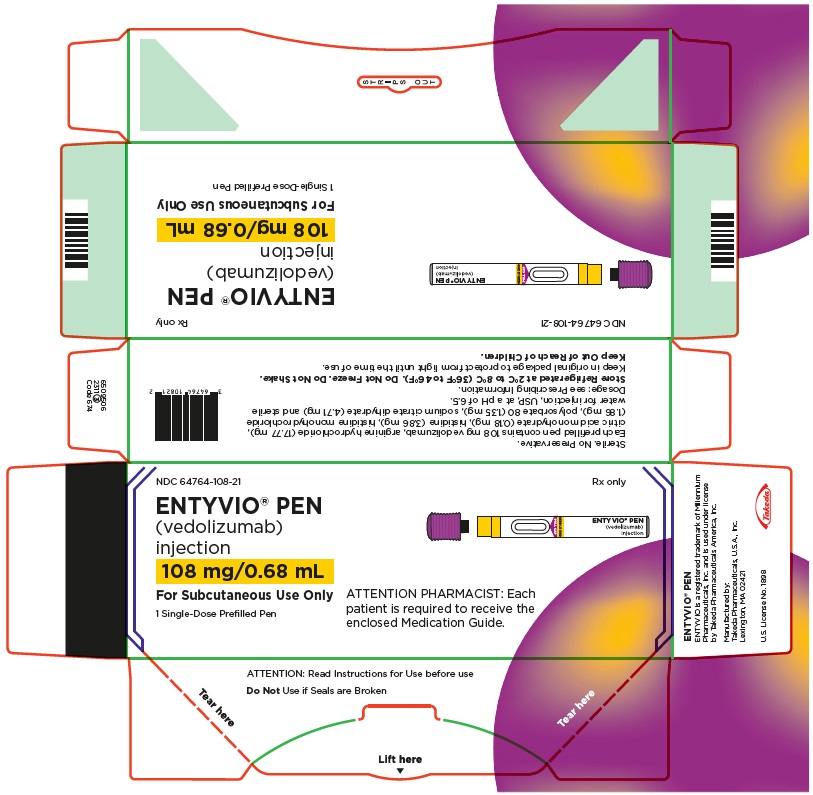
Single-dose prefilled pen
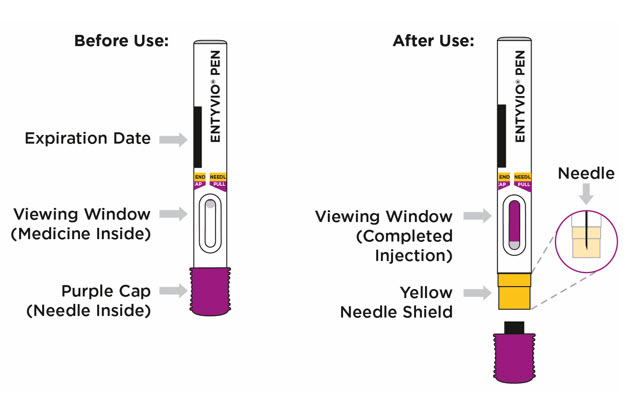
- Store your prefilled pen in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Your prefilled pen can be left in its box at room temperature up to 77°F (25°C) for up to 7 days (for example, when traveling).
- Do not freeze the prefilled pen.
- Do not leave the prefilled pen in direct sunlight.
- Throw away the prefilled pen in a FDA-cleared sharps disposal container if it has been left out of the refrigerator for more than 7 days, frozen, or left in direct sunlight. See
- Always keep ENTYVIO PENs, the sharps disposal container, and all medicines out of the reach of children.
Single-dose prefilled syringe
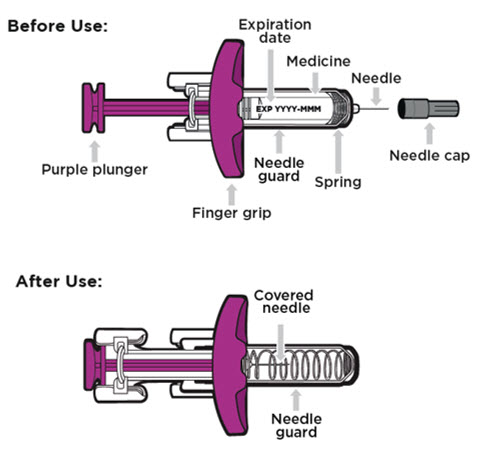
- Store your prefilled syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Your prefilled syringe can be left in its box at room temperature up to 77°F (25°C) for up to 7 days (for example, when traveling).
- Do not freeze the prefilled syringe.
- Do not leave the prefilled syringe in direct sunlight.
- Throw away the prefilled syringe in a FDA-cleared sharps disposal container if it has been left out of the refrigerator for more than 7 days, frozen, or left in direct sunlight. See
- Always keep ENTYVIO prefilled syringes, the sharps disposal container, and all medicines out of the reach of children.
vedolizumab
300 mg per vial*

vedolizumab
300 mg per vial*
(vedolizumab)
injection
(vedolizumab)
injection