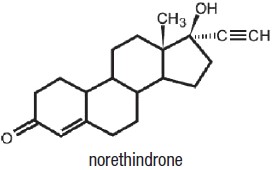
Norethindrone Acetate
What is Sharobel (Norethindrone Acetate)?
Approved To Treat
Related Clinical Trials
Summary: This is a prospective, open-label, multi-center seamless phase II to phase III randomized clinical trial designed to compare SST with or without PET-directed local therapy in improving the castration-resistant prostate cancer-free survival (CRPC-free survival) for Veterans with oligometastatic prostate cancer. Oligometastasis will be defined as 1-10 sites of metastatic disease based on the clinica...
Summary: The purpose of the study is to assess and evaluate dosimetry, safety, and tolerability following administration of up to 12 cycles of (177Lu) vipivotide tetraxetan (also referred to as \[177Lu\]Lu-PSMA-617 or 177Lu-PSMA-617 and hereafter identified as AAA617) in taxane-naïve adult participants with PSMA-positive mCRPC who progressed on a prior ARPI treatment with normal renal function or mild rena...
Summary: This phase II trial compares the effect of relugolix to leuprolide on cardiac function and performance in patients with prostate cancer. Androgen deprivation therapy (ADT) has been a key component for the treatment of advanced prostate cancer for decades. The term androgen deprivation therapy means lowering a man's testosterone. Long-term studies show that ADT may contribute to a detriment to card...
Related Latest Advances
Brand Information
- Known or suspected pregnancy
- Known or suspected carcinoma of the breast
- Undiagnosed abnormal genital bleeding
- Hypersensitivity to any component of this product
- Benign or malignant liver tumors
- Acute liver disease
- Menstrual irregularity is the most frequently reported side effect.
- Frequent and irregular bleeding are common, while long duration of bleeding episodes and amenorrhea are less likely.
- Headache, breast tenderness, nausea, and dizziness are increased among progestin-only oral contraceptive users in some studies.
- Androgenic side effects such as acne, hirsutism, and weight gain occur rarely.
- It’s best to take your fi rst POP on the fi rst day of your menstrual period (Day 1 Start). If you use a Day 1 Start, you are protected from becoming pregnant as soon as you take your first pill.
- If you decide to take your first POP on another day, use a backup method (such as a condom and/or a spermicide) every time you have sex during the next 48 hours.
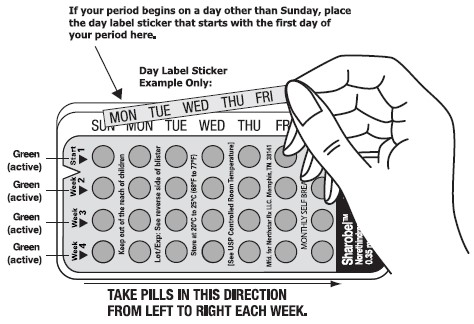
- Pick the day label sticker that starts with the fi rst day of your period.
- Place this day label sticker over the area on the plastic compact which already has the days of the week (starting with Sunday) imprinted and press firmly.
- To remove a tablet, i) ensure the blister is properly placed into the compact with the entire blister locked on the right V notch and placed under the six plastic lips of the compact, ii) press down the tablet with even pressure with your thumb or fi nger. The tablet will be pushed through the back of the compact tablet dispenser. Do not press with your thumbnail, fi ngernail, or any other sharp object.
- Swallow the pill. You will take one pill each day. POPs must be taken at the same time every day, so choose a time and then take the pill at that same time every day. Every time you take a pill late, and especially if you miss a pill, you are more likely to get pregnant.
- Wait 24 hours to take your next pill. POPs must be taken at the same time every day, so choose a time and then take the pill at that same time every day. Every time you take a pill late, and especially if you miss a pill, you are more likely to get pregnant. Continue to take one pill each day whether bleeding or not until all the pills have been taken.
- Take your pill at the same time every day. It is important to take the correct pill eachday and not miss any pills. To help you remember, take your pill at the same time as another daily activity, like turning off your alarm clock or brushing your teeth.
- You will start a new blister pack on the day after your blister pack is empty.
- THE FIRST PILL IN EVERY BLISTER PACK WILL ALWAYS BE TAKEN ON THE SAME DAY OF THE WEEK, NO MATTER WHEN YOUR NEXT PERIOD STARTS.
- If you are more than 3 hours late or you miss one or more POPs:
- If you are not sure what to do about the pills you have missed, keep taking POPs and use a backup method until you can talk to your healthcare professional.
- POPs must be taken at the same time every day, so choose a time and then take the pill at that same time every day. Every time you take a pill late, and especially if you miss a pill, you are more likely to get pregnant.
- Start the next pack the day after the last pack is finished. There is no break between packs. Always have your next pack of pills ready.
- You may have some menstrual spott ing between periods. Do not stop taking your pills if this happens.
- If you vomit soon after taking a pill, use a backup method (such as a condom and/or a spermicide) for 48 hours.
- If you want to stop taking POPs, you can do so at any time, but, if you remain sexually active and don’t wish to become pregnant, be certain to use another birth control method.
- If you are not sure about how to take POPs, ask your healthcare professional.