Brand Name
Nipent
Generic Name
Pentostatin
View Brand Information FDA approval date: August 15, 2007
Classification: Nucleoside Metabolic Inhibitor
Form: Injection
What is Nipent (Pentostatin)?
NIPENT is indicated as single-agent treatment for both untreated and alpha-interferon-refractory hairy cell leukemia patients with active disease as defined by clinically significant anemia, neutropenia, thrombocytopenia, or disease-related symptoms.
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
Pilot Trial of Allogeneic Blood or Marrow Transplantation for Primary Immunodeficiencies
Background: Allogeneic blood or marrow transplant is when stem cells are taken from one person s blood or bone marrow and given to another person. Researchers think this may help people with immune system problems.
Phase II Trial of Allogeneic Hematopoietic Cell Transplantation for Peripheral T Cell Lymphoma
Background: Lymphoma is a type of blood cancer. Blood cell transplant can cure some people with lymphoma. Researchers want to see if they can limit the complications transplant can cause.
A Phase I/II Trial of Allogeneic Reduced-Intensity, HLA-Haploidentical Transplantation Followed by GVHD Prophylaxis With Cyclophosphamide, Bortezomib and Maraviroc for Hematologic Malignancies in People Living With HIV (PLWH)
Background: People living with HIV(PLWH) are at a higher risk for cancers that may be curable with a bone marrow transplant. HIV infection itself is no longer a reason to not get a transplant, for patients who otherwise have a standard reason to need transplant.
Related Latest Advances
Brand Information
Nipent (PENTOSTATIN)
WARNING
NIPENT should be administered under the supervision of a physician qualified and experienced in the use of cancer chemotherapeutic agents. The use of higher doses than those specified (see
In a clinical investigation in patients with refractory chronic lymphocytic leukemia using NIPENT at the recommended dose in combination with fludarabine phosphate, 4 of 6 patients entered in the study had severe or fatal pulmonary toxicity. The use of NIPENT in combination with fludarabine phosphate is not recommended.
1DESCRIPTION
NIPENT
Pentostatin, also known as 2'-deoxycoformycin (DCF), is a potent inhibitor of the enzyme adenosine deaminase and is isolated from fermentation cultures of
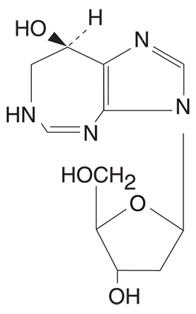
Pentostatin is a white to off-white solid, freely soluble in distilled water.
2CLINICAL PHARMACOLOGY
Mechanism of Action
Pentostatin is a potent transition state inhibitor of the enzyme adenosine deaminase (ADA). The greatest activity of ADA is found in cells of the lymphoid system with T-cells having higher activity than B-cells, and T-cell malignancies having higher ADA activity than B-cell malignancies. Pentostatin inhibition of ADA, particularly in the presence of adenosine or deoxyadenosine, leads to cytotoxicity, and this is believed to be due to elevated intracellular levels of dATP which can block DNA synthesis through inhibition of ribonucleotide reductase. Pentostatin can also inhibit RNA synthesis as well as cause increased DNA damage. In addition to elevated dATP, these mechanisms may also contribute to the overall cytotoxic effect of pentostatin. The precise mechanism of pentostatin's antitumor effect, however, in hairy cell leukemia is not known.
Pharmacokinetics/Drug Metabolism
A tissue distribution and whole-body autoradiography study in the rat revealed that radioactivity concentrations were highest in the kidneys with very little central nervous system penetration.
In man, following a single-dose of 4 mg/m
A positive correlation was observed between pentostatin clearance and creatinine clearance (CL
3CLINICAL STUDIES
The following table provides efficacy results for 4 groups (columns) of patients with hairy cell leukemia: patients who initially received NIPENT, patients who initially received alpha-interferon (IFN), and 2 different groups of patients who received NIPENT after proving to be refractory to, or intolerant of IFN therapy. The first 2 groups represent treatment results from the SWOG 8691 study, a large multicenter study comparing NIPENT and IFN in untreated (frontline) patients with confirmed hairy cell leukemia. The third group represents evaluable patients from the SWOG study who crossed over to NIPENT after initially receiving IFN. The fourth group, labeled NCI Phase 2 studies, displays pooled results of 2 noncomparative studies (MD Anderson and CALGB), in which NIPENT was used to treat patients with confirmed IFN-refractory disease.
In the SWOG 8691 study, NIPENT was administered at a dose of 4 mg/m
Interferon-refractory patients enrolled into the MD Anderson study received NIPENT at a dose of 4 mg/m
For each study, a complete response (CR) required clearing of the peripheral blood and bone marrow of all hairy cells, normalization of organomegaly and lymphadenopathy by physical examination, and recovery of hemoglobin to at least 12 g/dL, platelet count to at least 100,000/mm
The results show that frontline patients treated with NIPENT achieved a significantly higher rate of response than those treated with IFN. The time to recovery of neutrophil and platelet counts was shorter with NIPENT treatment and the estimated duration of response was longer. The response rate in IFN-refractory patients treated with NIPENT was similar to that in NIPENT-treated frontline patients. At a median follow-up duration of 46 months, there was no statistically significant difference in survival between hairy cell leukemia patients initially treated with NIPENT and those initially treated with IFN. However, no definite conclusions regarding survival can be made from these results because they are complicated by the fact that the majority of IFN patients crossed over to NIPENT treatment.
In the Phase 3 SWOG study, 25 patients with hairy cell leukemia died during treatment or follow-up: 18 patients had last received NIPENT (3 of whom had crossed over from IFN), and 7 patients had last received IFN (1 of whom crossed over from NIPENT). Eleven of the 25 deaths occurred within 60 days of the last dose of treatment. Of these, hairy cell leukemia was cited by the investigators as a contributory cause for 1 death in the NIPENT group and 3 deaths in the IFN group. Additionally, infection contributed to the deaths of 3 patients in the NIPENT group and 2 patients in the IFN group. Approximately 4% of hairy cell leukemia patients, in each arm, died more than 60 days after the last dose of either treatment and there was no outstanding cause of death among these patients.
4INDICATIONS AND USAGE
NIPENT is indicated as single-agent treatment for both untreated and alpha-interferon-refractory hairy cell leukemia patients with active disease as defined by clinically significant anemia, neutropenia, thrombocytopenia, or disease-related symptoms.
5CONTRAINDICATIONS
NIPENT is contraindicated: In patients who have demonstrated hypersensitivity to NIPENT.
6WARNINGS
See
Patients with hairy cell leukemia may experience myelosuppression primarily during the first few courses of treatment. Patients with infections prior to NIPENT treatment have in some cases developed worsening of their condition leading to death, whereas others have achieved complete response. Patients with infection should be treated only when the potential benefit of treatment justifies the potential risk to the patient. Efforts should be made to control the infection before treatment is initiated or resumed.
In patients with progressive hairy cell leukemia, the initial courses of NIPENT treatment were associated with worsening of neutropenia. Therefore, frequent monitoring of complete blood counts during this time is necessary. If severe neutropenia continues beyond the initial cycles, patients should be evaluated for disease status, including a bone marrow examination.
Elevations in liver function tests occurred during treatment with NIPENT and were generally reversible.
Renal toxicity was observed at higher doses in early studies; however, in patients treated at the recommended dose, elevations in serum creatinine were usually minor and reversible. There were some patients who began treatment with normal renal function who had evidence of mild to moderate toxicity at a final assessment (See
Rashes, occasionally severe, were commonly reported and may worsen with continued treatment. Withholding of treatment may be required (See
Acute pulmonary edema and hypotension, leading to death, have been reported in the literature in patients treated with pentostatin in combination with carmustine, etoposide and high dose cyclophosphamide as part of the ablative regimen for bone marrow transplant.
Pregnancy
Pentostatin can cause fetal harm when administered to a pregnant woman. Pentostatin was administered intravenously at doses of 0, 0.01, 0.1, or 0.75 mg/kg/day (0, 0.06, 0.6, and 4.5 mg/m
7ADVERSE REACTIONS
Most patients treated for hairy cell leukemia in the five NCI-sponsored Phase 2 studies and the Phase 3 SWOG study experienced an adverse event. The following table lists the most frequently occurring adverse events in patients treated with NIPENT (both frontline and IFN-refractory patients) compared with IFN (frontline only), regardless of drug association. The drug association of some adverse events is uncertain as they may be associated with the disease itself (e.g., infection, hematologic suppression), but other events, such as the gastrointestinal symptoms, rashes, and abnormal liver function tests, can in many cases be attributed to the drug. Most adverse events that were assessed for severity were either mild or moderate, and diminished in frequency with continued therapy.
The total incidence for all types of infections is considerably higher for both treatment groups in the SWOG 8691 study than is listed in the table above. An intent-to-treat analysis of infections found that 38% of patients treated with NIPENT and 34% of patients treated with IFN averaged 2.4 and 1.9 documented infections during treatment, respectively. The following table lists the different types of infections that were reported as adverse events during the initial phase of the SWOG study. There were no apparent differences in the types of infection between the 2 treatment groups, with the possible exception of herpes zoster which was reported more frequently for NIPENT (8%) than for IFN (1%).
The drug relatedness of the adverse events listed below cannot be excluded. The following adverse events occurred in 3% to 10% of NIPENT-treated patients in the initial phase of the SWOG study:
Body as a Whole—Chest Pain, Death, Face Edema, Peripheral Edema
Cardiovascular System—Hemorrhage, Hypotension
Digestive System—Dental Abnormalities, Dyspepsia, Flatulence, Gingivitis
Hematologic System—Agranulocytosis
Laboratory Deviations—Elevated Creatinine
Musculoskeletal System—Arthralgia
Nervous System—Confusion, Dizziness, Insomnia, Paresthesia, Somnolence
Psychobiologic Function—Anxiety, Depression, Nervousness
Respiratory System—Asthma
Skin & Appendages—Skin Dry, Urticaria
The remaining adverse events which occurred in less than 3% of NIPENT-treated patients during the initial phase of the SWOG study:
Body as a Whole—Flu-like Symptoms, Hangover Effect, Neoplasm
Cardiovascular System—Angina Pectoris, Arrhythmia, A-V Block, Bradycardia, Extrasystoles Ventricular, Heart Arrest, Heart Failure, Hypertension, Pericardial Effusion, Phlebitis, Pulmonary Embolus, Sinus Arrest, Tachycardia, Thrombophlebitis Deep, Vasculitis
Digestive System—Constipation, Dysphagia, Glossitis, Ileus
Hematologic System—Acute Leukemia, Anemia-Hemolytic, Aplastic Anemia
Laboratory Deviations—Hypercalcemia, Hyponatremia
Musculoskeletal System—Arthritis, Gout
Nervous System—Amnesia, Ataxia, Convulsions, Dreaming Abnormal, Dysarthria, Encephalitis, Hyperkinesia, Meningism, Neuralgia, Neuritis, Neuropathy, Paralysis, Syncope, Twitching, Vertigo
Psychobiologic Function—Decrease/Loss Libido, Emotional Lability, Hallucination, Hostility, Neurosis, Thinking Abnormal
Respiratory System—Bronchospasm, Larynx Edema
Skin and Appendages—Acne, Alopecia, Eczema, Petechial Rash, Photosensitivity Reaction
Special Senses—Amblyopia, Deafness, Earache, Eyes Dry, Labyrinthitis, Lacrimation Disorder, Nonreactive Eye, Photophobia, Retinopathy, Tinnitus, Unusual Taste, Vision Abnormal, Watery Eyes
Urogenital System—Amenorrhea, Breast Lump, Impotence, Kidney Function Abnormal, Nephropathy, Renal Failure, Renal Insufficiency, Renal Stone
One patient with hairy cell leukemia treated with NIPENT during another clinical study developed unilateral uveitis with vision loss.
Nineteen (5%) patients withdrew from the Phase 3 SWOG 8691 study because of adverse events; 9 during initial NIPENT treatment, 4 during NIPENT crossover, 5 during initial IFN treatment, and 1 during both initial IFN treatment and NIPENT crossover. In the Phase 2 studies in IFN-refractory hairy cell leukemia, 11% of patients withdrew from treatment with NIPENT due to an adverse event.
7.1Postmarketing Experience
The following adverse reactions have been identified during post-approval use of NIPENT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hematologic System—Febrile Neutropenia, Hemolytic Uremic Syndrome, Thrombotic Thrombocytopenic Purpura, Autoimmune Thrombocytopenia
Respiratory System—Acute Respiratory Failure
Skin and Appendages—Exfoliative Dermatitis
8OVERDOSAGE
No specific antidote for NIPENT overdose is known. NIPENT administered at higher doses (20- 50 mg/m
9DOSAGE AND ADMINISTRATION
It is recommended that patients receive hydration with 500 to 1,000 mL of 5% Dextrose in 0.5 Normal Saline or equivalent before NIPENT administration. An additional 500 mL of 5% Dextrose or equivalent should be administered after NIPENT is given.
The recommended dosage of NIPENT for the treatment of hairy cell leukemia is 4 mg/m
Higher doses are not recommended.
No extravasation injuries were reported in clinical studies.
The optimal duration of treatment has not been determined. In the absence of major toxicity and with observed continuing improvement, the patient should be treated until a complete response has been achieved. Although not established as required, the administration of two additional doses has been recommended following the achievement of a complete response.
All patients receiving NIPENT at 6 months should be assessed for response to treatment. If the patient has not achieved a complete or partial response, treatment with NIPENT should be discontinued.
If the patient has achieved a partial response, NIPENT treatment should be continued in an effort to achieve a complete response. At any time thereafter that a complete response is achieved, two additional doses of NIPENT are recommended. NIPENT treatment should then be stopped. If the best response to treatment at the end of 12 months is a partial response, it is recommended that treatment with NIPENT be stopped.
Withholding or discontinuation of individual doses may be needed when severe adverse reactions occur. Drug treatment should be withheld in patients with severe rash, and withheld or discontinued in patients showing evidence of nervous system toxicity.
NIPENT treatment should be withheld in patients with active infection occurring during the treatment but may be resumed when the infection is controlled.
Patients who have elevated serum creatinine should have their dose withheld and a CL
Patients with impaired renal function should be treated only when the potential benefit justifies the potential risk. Two patients with impaired renal function (CL
No dosage reduction is recommended at the start of therapy with NIPENT in patients with anemia, neutropenia, or thrombocytopenia. In addition, dosage reductions are not recommended during treatment in patients with anemia and thrombocytopenia if patients can be otherwise supported hematologically. NIPENT should be temporarily withheld if the absolute neutrophil count falls during treatment below 200 cells/mm
Preparation of Intravenous Solution
- Procedures for proper handling and disposal of anticancer drugs should be followed. Several guidelines on this subject have been published.
- Protective clothing including polyethylene gloves must be worn.
- Transfer 5 mL of Sterile Water for Injection USP to the vial containing NIPENT and mix thoroughly to obtain complete dissolution of a solution yielding 2 mg/mL. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
- NIPENT may be given intravenously by bolus injection or diluted in a larger volume (25 to 50 mL) with 5% Dextrose Injection USP or 0.9% Sodium Chloride Injection USP. Dilution of the entire contents of a reconstituted vial with 25 mL or 50 mL provides a pentostatin concentration of 0.33 mg/mL or 0.18 mg/mL, respectively, for the diluted solutions.
- NIPENT solution when diluted for infusion with 5% Dextrose Injection USP or 0.9% Sodium Chloride Injection USP does not interact with PVC infusion containers or administration sets at concentrations of 0.18 mg/mL to 0.33 mg/mL.
Stability
NIPENT vials are stable at refrigerated storage temperature 2° to 8° C (36° to 46°F) for the period stated on the package. Vials reconstituted or reconstituted and further diluted as directed may be stored at room temperature and ambient light but should be used within 8 hours because NIPENT contains no preservatives.
10HOW SUPPLIED
NIPENT (pentostatin for injection) is supplied as a sterile lyophilized white to off-white powder in single-dose vials containing 10 mg of pentostatin. The vials are packed in individual cartons.
Storage: Store NIPENT vials under refrigerated storage conditions 2° to 8° C (36° to 46°F).
11REFERENCES
- Malspeis L, et al. Clinical pharmacokinetics of 2'-Deoxycoformycin. Cancer Treatment Symposia 2:7-15, 1984
- Recommendations for the safe handling of parenteral antineoplastic drugs. NIH Publication 83-2621. For sale by the Superintendent of Documents, US Government Printing Office, Washington, DC 20402.
- AMA council Report. Guidelines for handling parenteral antineoplastics. JAMA 253:1590-2, 1985.
- National Study Commission on Cytotoxic Exposure—Recommendations for Handling Cytotoxic Agents. Available from Louis P. Jeffrey, Sc.D., Chairman, National Study Commission on Cytotoxic Exposure, Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, Massachusetts 02115.
- Clinical Oncological Society of Australia: Guidelines and recommendations for safe handling of antineoplastic agents. Med J Australia 1:426-8, 1983.
- Jones RB, et al. Safe handling of chemotherapeutic agents: A report from the Mount Sinai Medical Center. CA: A Cancer Journal for Clinicians 33:258-63, 1983.
- American Society of Hospital Pharmacists technical assistance bulletin on handling cytotoxic and hazardous drugs. Am J Hosp Pharm 47:1033-49, 1990.

Distributed by Hospira, Inc.
LAB-1220-4.0
Revised: 11/2025
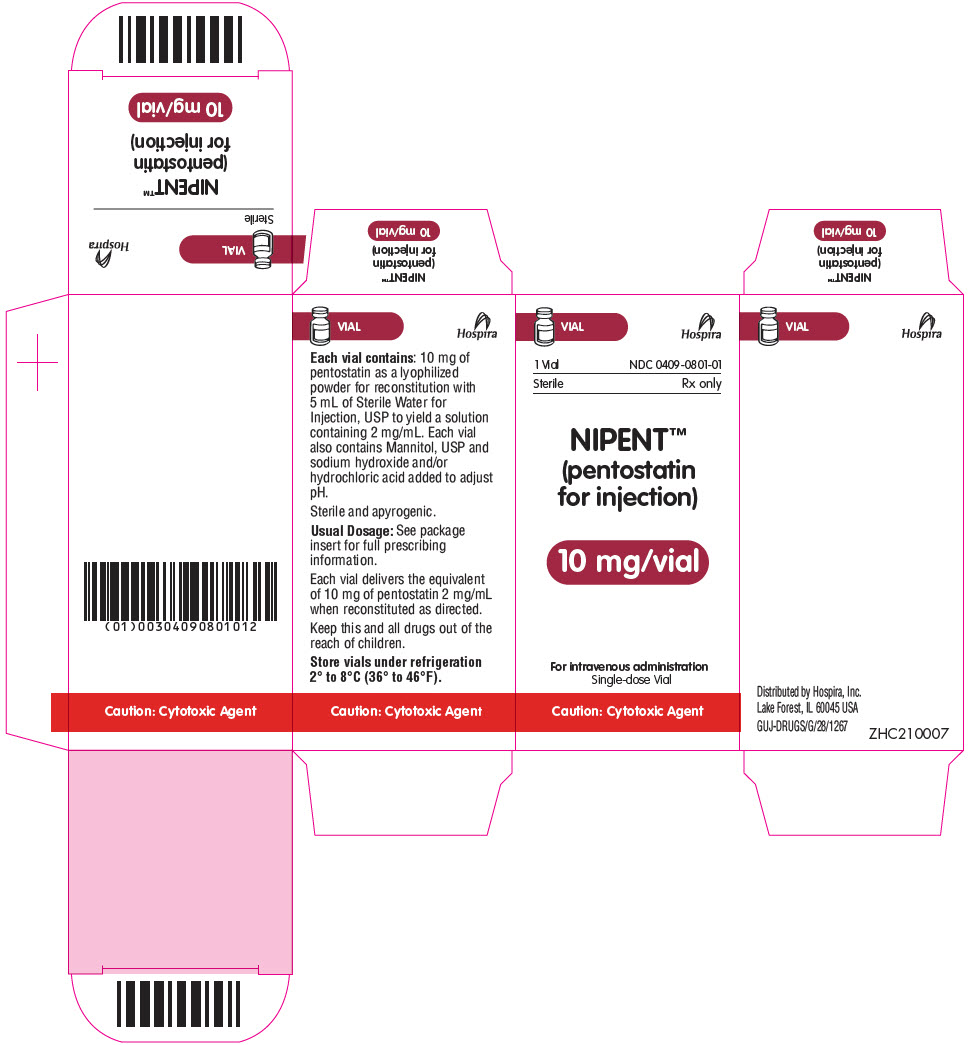
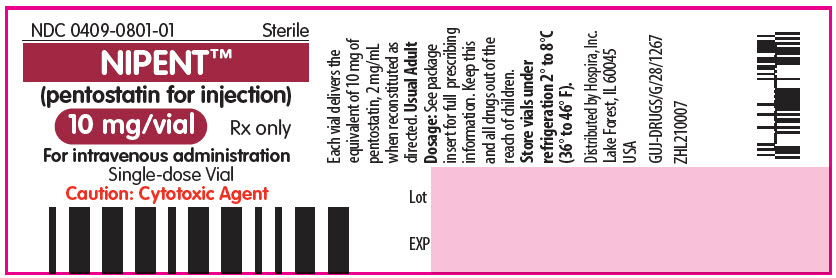
12PRINCIPAL DISPLAY PANEL - 10 mg Vial Label
NDC 0409-0801-01
NIPENT™
10 mg/vial
For intravenous administration
13PRINCIPAL DISPLAY PANEL - 10 mg Vial Carton
VIAL
1 Vial
Sterile
NIPENT™
10 mg/vial
For intravenous administration
Caution: Cytotoxic Agent