Mavenclad
What is Mavenclad (Cladribine)?
Approved To Treat
Related Clinical Trials
Summary: This phase I/II trial finds the best dose, side effects and how well giving venetoclax in combination with cladribine, cytarabine, granulocyte colony-stimulating factor, and mitoxantrone (CLAG-M) in treating patients with acute myeloid leukemia and high-grade myeloid neoplasms. Venetoclax may stop the growth of cancer cells by blocking Bcl-2, a protein needed for cancer cell survival. Chemotherapy...
Summary: This phase I/II trial tests the safety, side effects, and effectiveness of tovorafenib in combination with rituximab in patients with classical hairy cell leukemia (cHCL) that has come back after a period of improvement (recurrent) or that has not responded to previous treatment (refractory) and compares the effect of tovorafenib and rituximab to current standard treatment of cladribine and rituxi...
Summary: To learn about the safety and tolerability of study drug combinations in patients with relapsed/refractory, IDH1-mutated myeloid malignancies with a co-signaling mutation.
Related Latest Advances
Brand Information
- Malignancies
- Risk of Teratogenicity
- in patients with current malignancy
- in pregnant women and in women and men of reproductive potential who do not plan to use effective contraception during MAVENCLAD dosing and for 6 months after the last dose in each treatment course. May cause fetal harm
- in patients infected with the human immunodeficiency virus (HIV
- in patients with active chronic infections (e.g., hepatitis or tuberculosis)
- in patients with a history of hypersensitivity to cladribine
- in women intending to breastfeed on a MAVENCLAD treatment day and for 10 days after the last dose
- Malignancies
- Risk of Teratogenicity
- Lymphopenia
- Infections
- Hematologic Toxicity
- Graft-Versus-Host Disease With Blood Transfusion
- Liver Injury
- Hypersensitivity
- Cardiac Failure
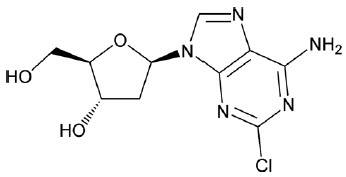
- "OSHA Hazardous Drugs". OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.