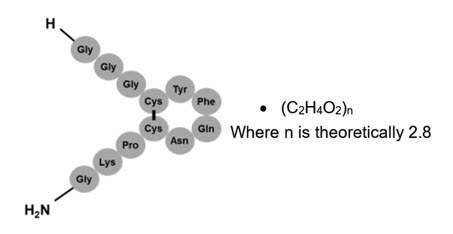
Terlivaz
What is Terlivaz (Terlipressin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This 2-part study will evaluate PHIN-214 given as a single one-time dose (Part 1) and in multiple doses (given as daily doses for 28-days) (in Part 2). Specifically, this study evaluates PHIN-214, to determine the safety, tolerability, and pharmacokinetic effects of PHIN-214, and to establish the maximum tolerated dose or optimal beneficial dose in patients with Child Pugh A and B Cirrhosis.
Summary: Prospective double blind randomized controlled trial. By randomizing patients undergoing kidney transplantation into a conventional catecholamine drug (dobutamine) blood pressure maintenance group and a terlipressin-complexed dobutamine group, the investigators compared the effect of intraoperative blood pressure maintenance and the dosage of the vasoactive drug, postoperative graft function, dela...
Summary: Patients with advanced cirrhosis of the liver develop kidney problems occasionally. This condition is called Hepatorenal Syndrome, requires hospitalization and frequently results in death. The goal of this clinical trial is to test whether the administration of low doses of ambrisentan can help patients with Hepatorenal Syndrome and to determine if it is safe. Ambrisentan is a drug that is approve...
Related Latest Advances
Brand Information
- Serious or Fatal Respiratory Failure
- Ischemic Events