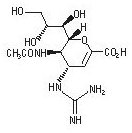
Relenza
What is Relenza (Zanamivir)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: ZAP-DENGUE is a pilot randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of five days of intravenous zanamivir treatment to treat vascular permeability syndrome which is the main cause of death in dengue fever.
Summary: Influenza infection is an important public health priority, with seasonal outbreaks and pandemics causing considerable global morbidity and mortality. The PK, pharmacodynamics (PD), safety and efficacy of IV zanamivir have been evaluated in adults, adolescents and infants more than or equal to (\>=) 6 months of age with hospitalized influenza in the IV zanamivir global development program. However...
Summary: This study is designed to help us better understand how the immune system responds to the flu and how flu is transmitted in the environment. The ultimate goal is to develop better vaccines and drugs to protect against or fight the flu. This study will describe how the body's immune system responds to the flu virus during and after infection and how the flu virus is transmitted in the environment. ...
Related Latest Advances
Brand Information
- Bronchospasm
- Safety information in patients with underlying airways disease
- Allergic-like reactions