Fintepla
What is Fintepla (Fenfluramine)?
Living with severe epilepsy, especially when seizures are frequent and unpredictable, can be overwhelming for both patients and caregivers. For children with rare forms of epilepsy like Dravet syndrome or Lennox-Gastaut syndrome (LGS), daily life often revolves around managing seizures, preventing injuries, and seeking moments of normalcy. Fintepla (fenfluramine) is an FDA-approved medication that helps reduce seizure frequency in these conditions, offering hope for better control and improved quality of life.
Fintepla is an anti-seizure medication (anticonvulsant) that works through a unique mechanism involving serotonin signaling in the brain. Originally developed decades ago for another purpose, it has been repurposed and rigorously studied to provide safe and effective seizure control in certain severe epileptic disorders. It is typically prescribed as an adjunct therapy used in combination with other anti-seizure medicines to help patients who do not respond adequately to standard treatments.
What does Fintepla do?
Fintepla is used to reduce the frequency and severity of seizures in patients with Dravet syndrome and Lennox-Gastaut syndrome, two rare and hard-to-treat forms of epilepsy that usually begin in early childhood.
Dravet syndrome is known for frequent, prolonged seizures that can be triggered by fever, light, or excitement. Lennox-Gastaut syndrome often causes multiple types of seizures, developmental delays, and cognitive challenges. Both conditions can significantly affect daily functioning and quality of life.
By adding Fintepla to a patient’s existing seizure medication regimen, many experience fewer and shorter seizures, as well as improvements in alertness and overall daily activity.
Clinical trials have shown that Fintepla can reduce monthly seizure frequency by up to 50% or more in many patients with Dravet syndrome, and it has also demonstrated meaningful improvements for those with LGS (FDA, 2023). For families affected by these challenging disorders, even a partial reduction in seizures can translate into better sleep, increased independence, and enhanced emotional well-being.
How does Fintepla work?
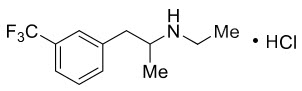
Fintepla contains fenfluramine, a medication that affects serotonin, a chemical messenger in the brain involved in regulating mood, sleep, and neurological activity. In epilepsy, irregular electrical activity causes seizures. Fintepla helps stabilize brain signaling by increasing serotonin’s influence on specific receptors that modulate nerve activity.
In simpler terms, the drug helps “calm down” overactive brain circuits that trigger seizures. Researchers believe fenfluramine works by:
- Enhancing serotonin transmission, which reduces hyperexcitability in the brain.
- Interacting with sigma-1 receptors, which may protect neurons and improve seizure control.
This dual action helps regulate abnormal brain activity without suppressing normal function.
Clinically, this mechanism is significant because Fintepla works differently from most traditional anti-seizure drugs. This makes it especially valuable for patients who have not responded well to other medications. However, due to its potent effects, it must be used under close medical supervision with regular safety monitoring.
Fintepla side effects
While Fintepla can be highly effective, it may also cause side effects. Most are mild to moderate, but some require careful medical attention.
Common side effects include:
- Decreased appetite and weight loss
- Fatigue or sleepiness
- Diarrhea or constipation
- Increased drooling or salivation
- Behavioral changes, such as irritability
Serious side effects (less common) include:
- Heart valve problems or pulmonary hypertension (increased pressure in the lungs)
- Depression or suicidal thoughts
- Low sodium levels in the blood (hyponatremia)
- Coordination or balance issues
Due to fenfluramine’s historical link to heart complications at high doses, Fintepla patients are enrolled in a REMS program requiring regular heart monitoring via echocardiograms to detect early valve or pressure changes. Parents or caregivers should immediately contact their doctor if the patient experiences shortness of breath, chest pain, fainting, or leg swelling, as these may indicate heart issues.
Fintepla should not be used by individuals taking monoamine oxidase inhibitors (MAOIs) or those with a history of heart valve disease or severe pulmonary hypertension. Your doctor will review your medical history carefully before starting treatment.
Fintepla dosage
Fintepla, a liquid oral solution, is taken by mouth, typically once or twice daily. Dosing is carefully adjusted to control seizures with minimal side effects and should never be changed or stopped without a doctor’s consultation. It can be taken with or without food, using a syringe for precise measurement.
Doctors monitor heart function with echocardiograms, appetite and weight, and seizure frequency. Dosage adjustments may be needed for those with liver or kidney issues. Regular follow-ups are crucial.
Does Fintepla have a generic version?
As of 2025, Fintepla (fenfluramine) does not have an FDA-approved generic version available in the United States. It is marketed exclusively under the brand name Fintepla, manufactured by UCB Pharma. However, international versions may exist in other markets.
Fintepla is currently only available through certified pharmacies in the REMS program until its patent expires and generic versions become available. Generics must meet the same FDA standards. Patients can seek assistance for treatment costs.
Conclusion
Fintepla represents a major advancement in the treatment of Dravet syndrome and Lennox-Gastaut syndrome, offering meaningful seizure reduction for patients who previously had few options. By targeting brain serotonin pathways, it helps stabilize nerve activity and improve seizure control, leading to better daily functioning and overall quality of life.
Though requiring heart monitoring and medical supervision, Fintepla generally offers more benefits than risks for severe, treatment-resistant epilepsies. Optimal dosage and therapy combinations may take time, but with proper use and follow-up, Fintepla can bring stability and hope to families managing epilepsy.
References
- U.S. Food and Drug Administration (FDA). (2023). Fintepla (fenfluramine) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Fenfluramine (oral route) drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Fenfluramine: Uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Antiseizure medications in Dravet and Lennox-Gastaut syndromes. Retrieved from https://www.nih.gov
Approved To Treat
Related Clinical Trials
Summary: Dravet syndrome is a genetic epilepsy associated with pathogenic variants in SCN1A that codes for Nav1.1, a protein necessary for sodium channels. Children with Dravet syndrome classically present in the first year of life with prolonged seizures, often hemiclonic and in the setting of fever or temperature changes such as getting in or out of bath water. Many anti-seizure medications are sodium ch...
Summary: This study is a pilot non-controlled clinical trial with adjunctive fenfluramine for the treatment of five different types of developmental and epileptic encephalopathies (DEEs) focused on epileptic and non-epileptic outcomes: SYNGAP1 and STXBP1 encephalopathies, inv-dup(15) encephalopathy, multifocal or bilateral malformations of cortical development, and continuous spikes and waves during sleep....
Summary: This is a phase II clinical trial in which children with refractory infantile spasms (also called epileptic spasms or West syndrome) will be treated with fenfluramine, to evaluate efficacy, safety, and tolerability. Patients with infantile spasms that have not responded to treatment with vigabatrin and ACTH we will be invited to participate. Study participants will undergo baseline video-EEG, rece...
Related Latest Advances
Brand Information
- Hypersensitivity to fenfluramine or any of the excipients in FINTEPLA
- Concomitant use, or within 14 days of the administration, of monoamine oxidase inhibitors because of an increased risk of serotonin syndrome
- Valvular Heart Disease and Pulmonary Arterial Hypertension
- Decreased Appetite and Decreased Weight
- Somnolence, Sedation, and Lethargy
- Suicidal Behavior and Ideation
- Withdrawal of Antiepileptic Drugs
- Serotonin Syndrome
- Increase in Blood Pressure
- Glaucoma
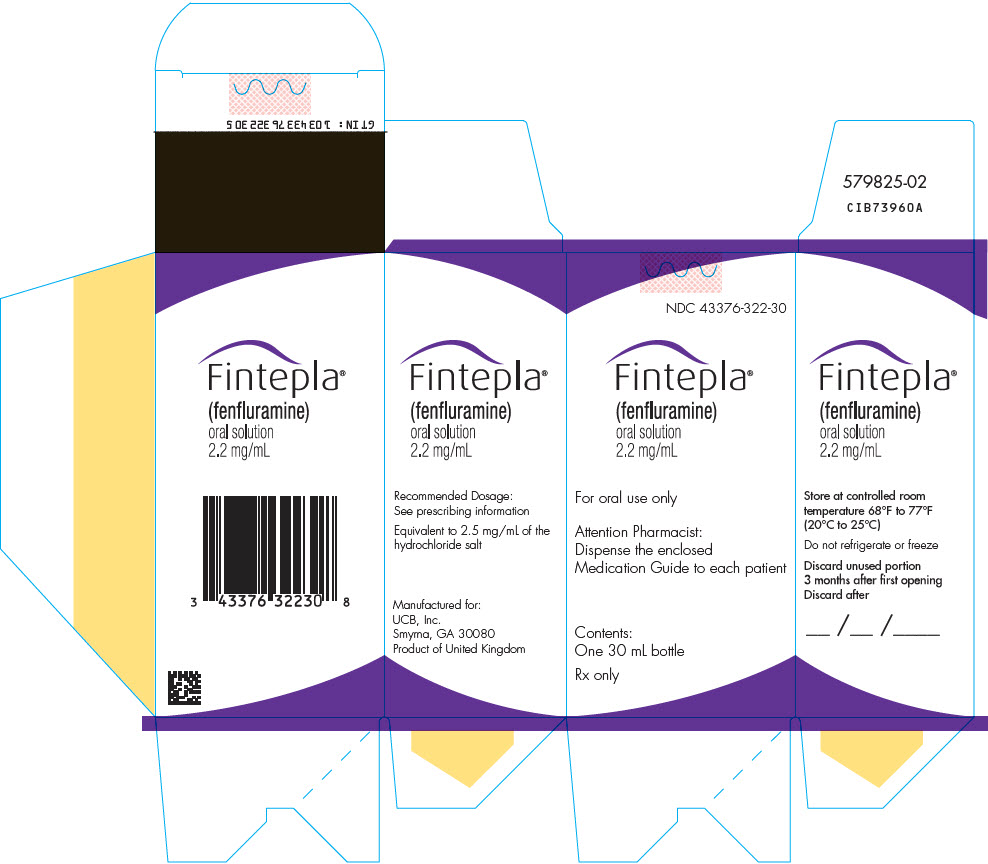
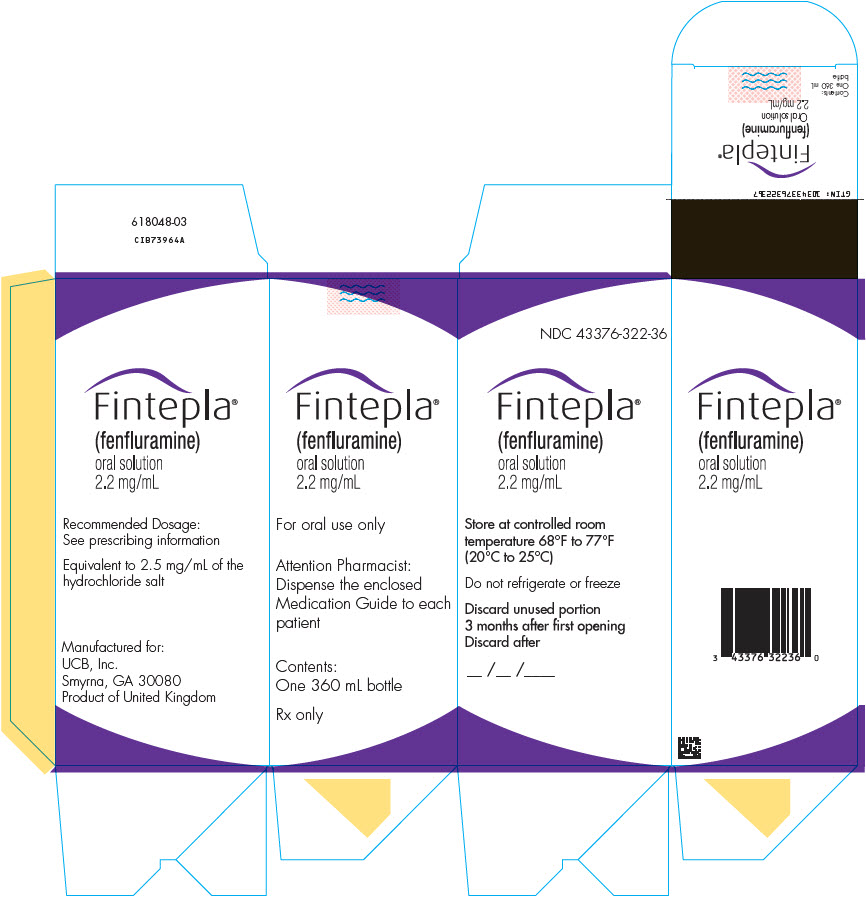
- 1 bottle of FINTEPLA oral solution (2.2 mg/mL)
- 2 reusable oral syringes
- FINTEPLA is an
- If you have questions about how to prepare or give FINTEPLA, contact your healthcare provider or call your pharmacist.
- Always use the oral syringes provided with FINTEPLA to make sure the right dose is given. If you need a new syringe contact your pharmacist.
- Store FINTEPLA at room temperature between 68°F to 77°F (20°C to 25°C).
- Do notrefrigerate or freeze.
- Keep the cap tightly closed and the bottle upright.
- Store the FINTEPLA bottle and syringe together in a clean area.
- Throw away (discard) any unused FINTEPLA 3 months after first opening the bottle
- Keep FINTEPLA and all medicines out of the reach of children.