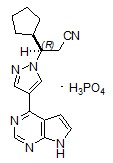
Ruxolitinib
What is Jakafi (Ruxolitinib)?
Living with a chronic blood disorder or severe skin condition can be physically and emotionally exhausting. Many people with these illnesses experience fatigue, itching, pain, or an enlarged spleen that affects daily life. Jakafi (ruxolitinib) is a prescription medication designed to help manage these symptoms and improve quality of life for patients facing conditions such as myelofibrosis, polycythemia vera, or graft-versus-host disease (GVHD) after stem cell transplant.
Jakafi belongs to a class of drugs known as Janus kinase (JAK) inhibitors. It works by targeting specific enzymes involved in inflammation and abnormal cell signaling within the body. Since its approval by the U.S. Food and Drug Administration (FDA) in 2011, Jakafi has become a specialized treatment that provides meaningful relief for patients who previously had limited options. For many, it represents a turning point in managing serious, long-term conditions that disrupt everyday health and well-being.
What does Jakafi do?
Jakafi is prescribed to treat several chronic and serious conditions related to abnormal immune or bone marrow function:
- Myelofibrosis (MF): A rare bone marrow disorder that causes scarring and disrupts the body’s ability to produce blood cells. Jakafi helps reduce spleen size, ease symptoms such as fatigue and night sweats, and improve energy levels.
- Polycythemia vera (PV): A condition in which the bone marrow makes too many red blood cells, thickening the blood and increasing the risk of clots. Jakafi helps control red blood cell counts when other treatments like hydroxyurea, are not effective.
- Acute and chronic graft-versus-host disease (GVHD): A complication that can occur after a stem cell transplant, where the donor’s immune cells attack the recipient’s tissues. Jakafi helps calm this immune overreaction and reduce inflammation.
Clinical studies show that Jakafi can shrink an enlarged spleen and significantly reduce symptoms in many patients with myelofibrosis or polycythemia vera (NIH, 2024). Patients often report improved appetite, reduced itching, and a noticeable increase in energy, all of which contribute to a better quality of life.
How does Jakafi work?
Jakafi (ruxolitinib) works by blocking the activity of Janus kinase (JAK) enzymes, specifically JAK1 and JAK2 which are involved in controlling immune responses and blood cell production.
In healthy individuals, JAK enzymes help transmit chemical signals that tell the body when to make blood cells or release inflammatory substances. However, in conditions like myelofibrosis and polycythemia vera, these signals become overactive, causing the bone marrow to produce too many or abnormal cells and leading to inflammation and organ enlargement.
By inhibiting JAK1 and JAK2, Jakafi reduces abnormal cell signaling, helping normalize blood cell production and calm inflammation. This process helps relieve symptoms such as fatigue, fever, itching, and spleen discomfort.
Clinically, this mechanism is vital for GVHD patients as Jakafi suppresses harmful immune activity, interrupting disease progression and restoring immune and hematologic balance. It offers targeted immune control, preserving infection-fighting ability better than traditional therapies.
Jakafi side effects
Like most prescription medications, Jakafi can cause side effects. Many are mild and improve as the body adjusts, but some may require monitoring or medical attention.
Common side effects may include:
- Headache or dizziness
- Fatigue
- Easy bruising or minor bleeding
- Weight gain or swelling in hands and feet
- Diarrhea or constipation
Serious side effects (less common):
- Low blood cell counts (anemia, neutropenia, or thrombocytopenia)
- Increased risk of infections, including shingles or urinary tract infections
- Shortness of breath or unusual tiredness
- Unexplained bleeding or bruising
- Liver enzyme changes detected on blood tests
Due to Jakafi’s impact on bone marrow and immune function, regular blood tests are crucial for monitoring blood cell and platelet counts, allowing doctors to adjust doses for safe, effective treatment.
Avoid Jakafi if you have active infections, very low blood counts, or a ruxolitinib allergy. Seek immediate medical help for allergic reactions, infection signs (fever, chills), or severe fatigue with shortness of breath (anemia). Though generally well-tolerated, close medical supervision minimizes risks and maximizes benefits.
Jakafi dosage
Jakafi is a tablet taken orally, usually twice daily. Dosage is personalized based on the patient’s condition, blood tests, and treatment response. It can be taken with or without food, but consistency is key. Doctors typically start with a low dose and adjust it based on blood counts and side effects.
Doctors monitor CBC, liver/kidney function, and infection risk due to Jakafi’s impact on blood production and liver function. Dose adjustments may be necessary for older adults or those with kidney/liver issues. Never stop or change the dose without consulting a healthcare provider, as abrupt discontinuation can lead to symptom recurrence.
Does Jakafi have a generic version?
As of 2025, no generic version of Jakafi (ruxolitinib) is approved in the United States. It is available only as the brand-name medication manufactured by Incyte Corporation, with distribution by Novartis in some regions. However, international versions may exist in other markets.
Ruxolitinib cream (Opzelura) treats skin conditions like vitiligo and eczema. A generic Jakafi will meet FDA quality, safety, and effectiveness standards. Patients with cost concerns should consult their provider or pharmacist about assistance programs.
Conclusion
Jakafi (ruxolitinib) is a targeted oral therapy that has transformed the treatment landscape for myelofibrosis, polycythemia vera, and graft-versus-host disease. By blocking overactive JAK enzymes, it helps restore balance to the body’s immune and blood systems reducing symptoms, shrinking spleen size, and improving overall well-being.
Jakafi is generally safe and effective for most patients, offering stability and relief from difficult-to-control diseases. Patients should communicate openly with their healthcare team, follow dosing, and report unusual symptoms for careful management, which can restore comfort, energy, and hope.
References
- U.S. Food and Drug Administration (FDA). (2024). Jakafi (ruxolitinib) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Ruxolitinib: Drug information and side effects. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Ruxolitinib oral: Uses, warnings, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Targeted therapy for myelofibrosis and polycythemia vera. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is an open-label phase I study of fostamatinib in combination with ruxolitinib for the treatment of chronic GvHD with a suboptimal response to corticosteroids. The primary objective is to identify a minimum safe and biologically effective dose of fostamatinib when combined with standard of care ruxolitinib for the treatment of steroid refractory and steroid dependent cGVHD. The secondary obje...
Summary: The purpose of this study is to assess the efficacy and safety of ruxolitinib therapy in Chinese adults and adolescents (≥ 12 years old) with Grade II-IV steroid-refractory acute graft versus host disease (SR-aGvHD).
Summary: The purpose of this study is to evaluate the maximal use of ruxolitinib cream in adult and adolescent participants with hidradenitis suppurativa.
Related Latest Advances
Brand Information
- Thrombocytopenia, Anemia and Neutropenia
- Risk of Infection
- Symptom Exacerbation Following Interruption or Discontinuation of Treatment with Jakafi
- Non-Melanoma Skin Cancer
- Lipid Elevations
- Major Adverse Cardiovascular Events (MACE)
- Thrombosis
- Secondary Malignancies
- 25% of patients treated with Jakafi and 7% of patients treated with placebo developed newly occurring or worsening Grade 1 abnormalities in alanine transaminase (ALT). The incidence of greater than or equal to Grade 2 elevations was 2% for Jakafi with 1% Grade 3 and no Grade 4 ALT elevations.
- 17% of patients treated with Jakafi and 6% of patients treated with placebo developed newly occurring or worsening Grade 1 abnormalities in aspartate transaminase (AST). The incidence of Grade 2 AST elevations was < 1% for Jakafi with no Grade 3 or 4 AST elevations.
- 17% of patients treated with Jakafi and < 1% of patients treated with placebo developed newly occurring or worsening Grade 1 elevations in cholesterol. The incidence of Grade 2 cholesterol elevations was < 1% for Jakafi with no Grade 3 or 4 cholesterol elevations.
- Infections and Infestations: Herpes simplex virus reactivation and/or dissemination