Zynlonta
What is Zynlonta (Loncastuximab Tesirine)?
Receiving a diagnosis of aggressive lymphoma can be overwhelming bringing uncertainty, fatigue, and a long road of treatments that can deeply affect daily life. For patients whose cancer has returned or resisted prior therapies, new options like Zynlonta (loncastuximab tesirine-lpyl) offer renewed hope.
Zynlonta is an intravenous cancer medication used to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), a fast-growing type of non-Hodgkin lymphoma. It belongs to a class of drugs called antibody-drug conjugates (ADCs), an advanced form of targeted cancer therapy that combines the precision of an antibody with the power of chemotherapy.
Approved by the U.S. Food and Drug Administration (FDA) in 2021, Zynlonta represents an important step forward in personalized oncology care. It is typically prescribed for patients who have already undergone at least two prior lines of systemic therapy, making it a specialized treatment option for individuals with limited alternatives.
What does Zynlonta do?
Zynlonta is designed to control and reduce the growth of certain types of lymphoma cells. Specifically, it targets diffuse large B-cell lymphoma (DLBCL) that either did not respond to previous treatments or has come back after initial remission. This includes DLBCL not otherwise specified, and disease arising from indolent lymphomas.
For patients and caregivers, this means Zynlonta offers another chance at remission when other therapies have failed. In clinical trials, Zynlonta demonstrated meaningful results: many patients experienced tumor shrinkage, and some achieved complete remission, meaning no detectable cancer after treatment (FDA, 2024).
Patients experience improved symptom control, less fatigue, reduced lymph node swelling, and better breathing or energy leading to regained strength and quality of life.
How does Zynlonta work?
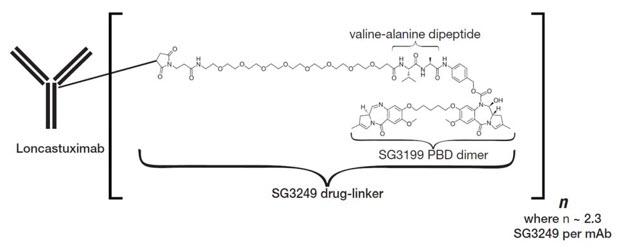
Zynlonta (loncastuximab tesirine-lpyl) is an antibody-drug conjugate (ADC), a sophisticated therapy that combines targeted precision with powerful cancer-killing action.
Here’s how it works:
- The antibody portion of Zynlonta specifically recognizes and attaches to CD19, a protein found on the surface of most B-cell lymphomas.
- Once attached, the ADC is absorbed into the cancer cell, carrying with it a cell-killing agent known as a cytotoxic payload.
- Inside the cell, this payload is released, damaging the DNA and ultimately leading to cancer cell death.
This dual mechanism allows Zynlonta to attack cancer cells directly while limiting harm to most healthy cells, an important distinction from traditional chemotherapy, which affects both cancerous and non-cancerous tissues.
Clinically, this targeted approach matters because it helps control disease progression with fewer systemic side effects than conventional chemotherapy, allowing many patients to continue treatment longer and with better tolerance.
Zynlonta side effects
As with all cancer treatments, Zynlonta can cause side effects that vary in intensity from person to person. Many are manageable with close medical supervision.
Common side effects include:
- Fatigue or low energy
- Swelling in the face or limbs (edema)
- Nausea or decreased appetite
- Skin rash or itching
- Low blood cell counts (anemia, low white blood cells, or platelets)
- Elevated liver enzymes (detected in blood tests)
Serious side effects may include:
- Infections: Because Zynlonta can reduce white blood cells, patients are more vulnerable to infections. Fever, chills, or persistent cough should be reported immediately.
- Liver toxicity: Signs include yellowing of the skin or eyes (jaundice), dark urine, or severe fatigue.
- Fluid retention: Excess fluid buildup can cause swelling, shortness of breath, or sudden weight gain.
- Photosensitivity: Skin can become more sensitive to sunlight; protective clothing and sunscreen are recommended during and for several months after treatment.
Inform your doctor about active infections, severe liver disease, or past severe allergic reactions to monoclonal antibodies before starting Zynlonta.
Seek emergency care for difficulty breathing, facial swelling, or rapid heartbeat after infusion, as these suggest a reaction. Doctors will monitor blood counts and liver function before each treatment.
Zynlonta dosage
Zynlonta is a potent intravenous (IV) infusion given in a hospital or cancer center by oncology specialists. Each 30-minute infusion is typically administered every three weeks. The medical team manages dosage and timing, adjusting or pausing therapy for significant side effects to ensure patient safety.
Before each infusion, patients undergo blood tests for liver function and cell counts, physical exams for side effects, and medication reviews to prevent interactions. Premedications like antihistamines or steroids may be recommended to minimize reactions. Patients are closely observed during and after infusion.
Older adults and those with mild-to-moderate liver impairment can receive Zynlonta with adjusted monitoring, but it should be avoided in severe hepatic dysfunction unless benefits outweigh risks.
Does Zynlonta have a generic version?
As of 2025, Zynlonta (loncastuximab tesirine-lpyl) does not have an FDA-approved generic version. It is available only as the brand-name product developed by ADC Therapeutics SA. However, international versions may exist in other markets.
Zynlonta, a biologic drug, will have biosimilar alternatives, not traditional generics. These biosimilars are clinically equivalent in safety and effectiveness. Currently, no loncastuximab biosimilars exist in the U.S. Patients can consult their oncologist or pharmacist about manufacturer assistance or insurance support for cost concerns.
Conclusion
Zynlonta represents a promising advancement for adults with difficult-to-treat or recurrent B-cell lymphoma. By targeting CD19-positive cancer cells and delivering a potent cancer-killing agent directly to them, it offers renewed hope where other treatments have stopped working.
Despite significant side effects and monitoring, Zynlonta offers substantial benefits, including disease control, remission, and improved survival for patients with limited prior options. Individual outcomes vary based on factors like prior treatments and overall health. Close collaboration with an oncology team, adherence to safety guidelines, and open communication can maximize Zynlonta’s benefits, empowering patients in their cancer journey.
References
- U.S. Food and Drug Administration (FDA). (2024). Zynlonta (loncastuximab tesirine-lpyl) prescribing information. Retrieved from https://www.accessdata.fda.gov
- MedlinePlus. (2024). Loncastuximab injection: Uses and side effects. National Library of Medicine. Retrieved from https://medlineplus.gov
- Mayo Clinic. (2024). Loncastuximab tesirine – Drug information. Retrieved from https://www.mayoclinic.org
- National Cancer Institute (NCI). (2024). Diffuse large B-cell lymphoma treatment (PDQ®)–Patient version. Retrieved from https://www.cancer.gov
Approved To Treat
Related Clinical Trials
Summary: This phase II trial studies the safety and how well of loncastuximab tesirine when given together with mosunetuzumab works in treating patients with diffuse large B-cell lymphoma that has come back (relapsed) or does not respond to treatment (refractory). Loncastuximab tesirine is a monoclonal antibody, loncastuximab, linked to a toxic agent called tesirine. Loncastuximab attaches to anti-CD19 can...
Summary: The purpose of this research study is to see if loncastuximab tesirine has any benefits at dose levels researchers found acceptable in earlier studies in patients with related forms of immune cell cancers. The researchers want to find out the effects (good and bad) that loncastuximab tesirine has on the participant and the participant's condition.
Summary: The primary objective of this study is to characterize the safety and tolerability of loncastuximab tesirine in combination with polatuzumab vedotin, glofitamab, or mosunetuzumab, and to identify the maximum tolerated dose (MTD) and/or recommended dose for expansion (RDE) for the combinations.
Related Latest Advances
Brand Information
- Effusion and Edema: Advise patients to contact their healthcare provider if they experience swelling, weight gain, shortness of breath, or difficult, labored breathing [see .
- Myelosuppression: Advise patients to immediately contact their healthcare provider for a fever of 100.4°F (38°C) or greater, or signs or symptoms of bruising or bleeding. Advise patients of the need for periodic monitoring of blood counts [see .
- Infections: Advise patients to contact their healthcare provider for signs or symptoms of infection, including fever, chills, weakness and/or difficulty breathing [see .
- Cutaneous Reactions: Advise patients that skin reaction or rash can occur. Patients should be directed to minimize or avoid exposure to direct natural or artificial sunlight, including sunlight exposure through glass windows. Patients should be instructed to protect skin from exposure to sunlight by wearing sun-protective clothing and/or the use of sunscreen products [see
- Embryo-Fetal Toxicity:
- Advise pregnant women of the potential risk to a fetus. Advise female patients of reproductive potential to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, during treatment with ZYNLONTA
- Advise women of reproductive potential to use effective contraception during treatment with ZYNLONTA and for 10 months after the last dose.
- Advise male patients with female partners of reproductive potential, to use effective contraception during treatment with ZYNLONTA and for 7 months after the last dose
- Lactation: Advise women not to breastfeed during treatment with ZYNLONTA and for 3 months after the last dose [see