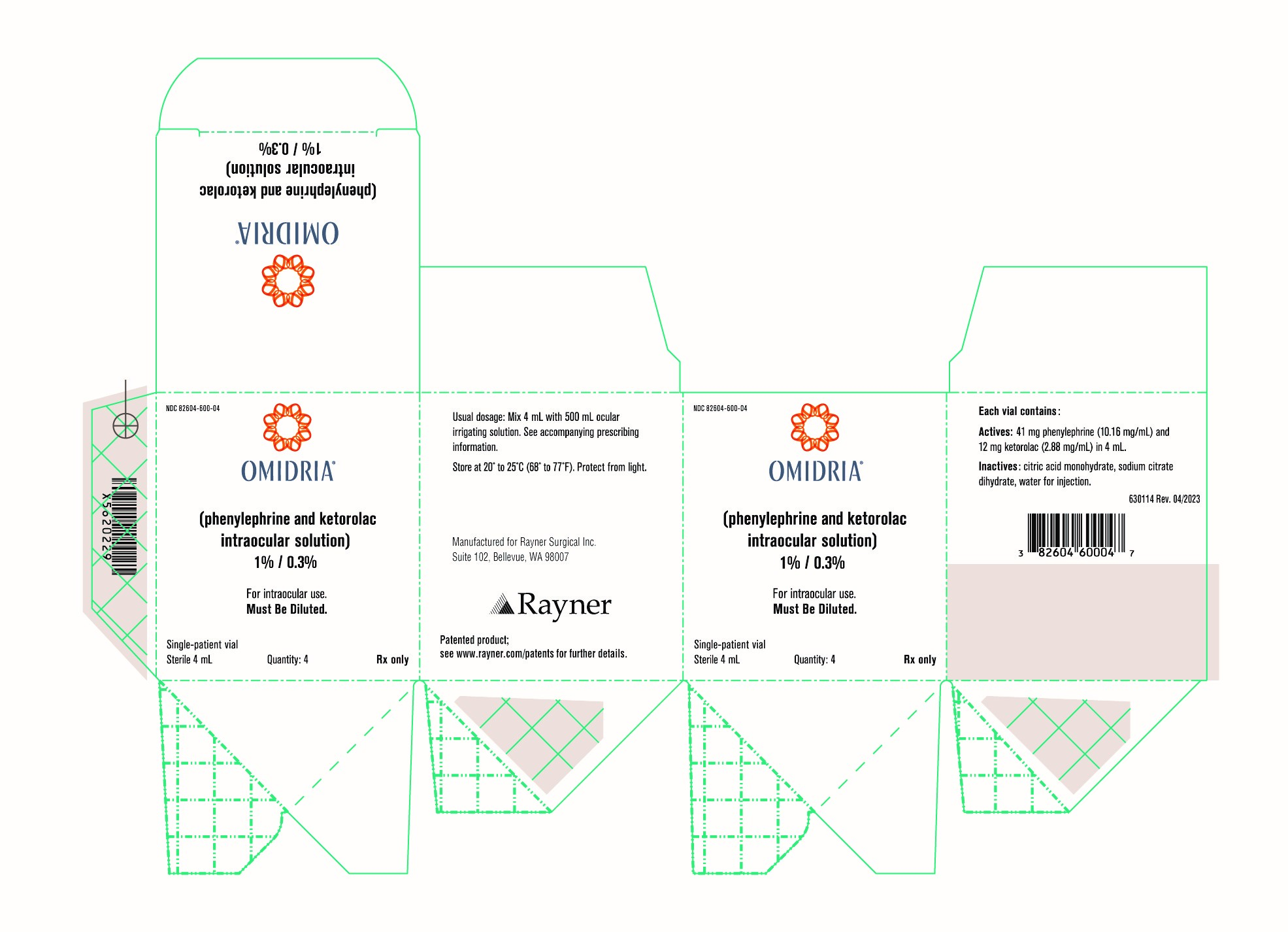
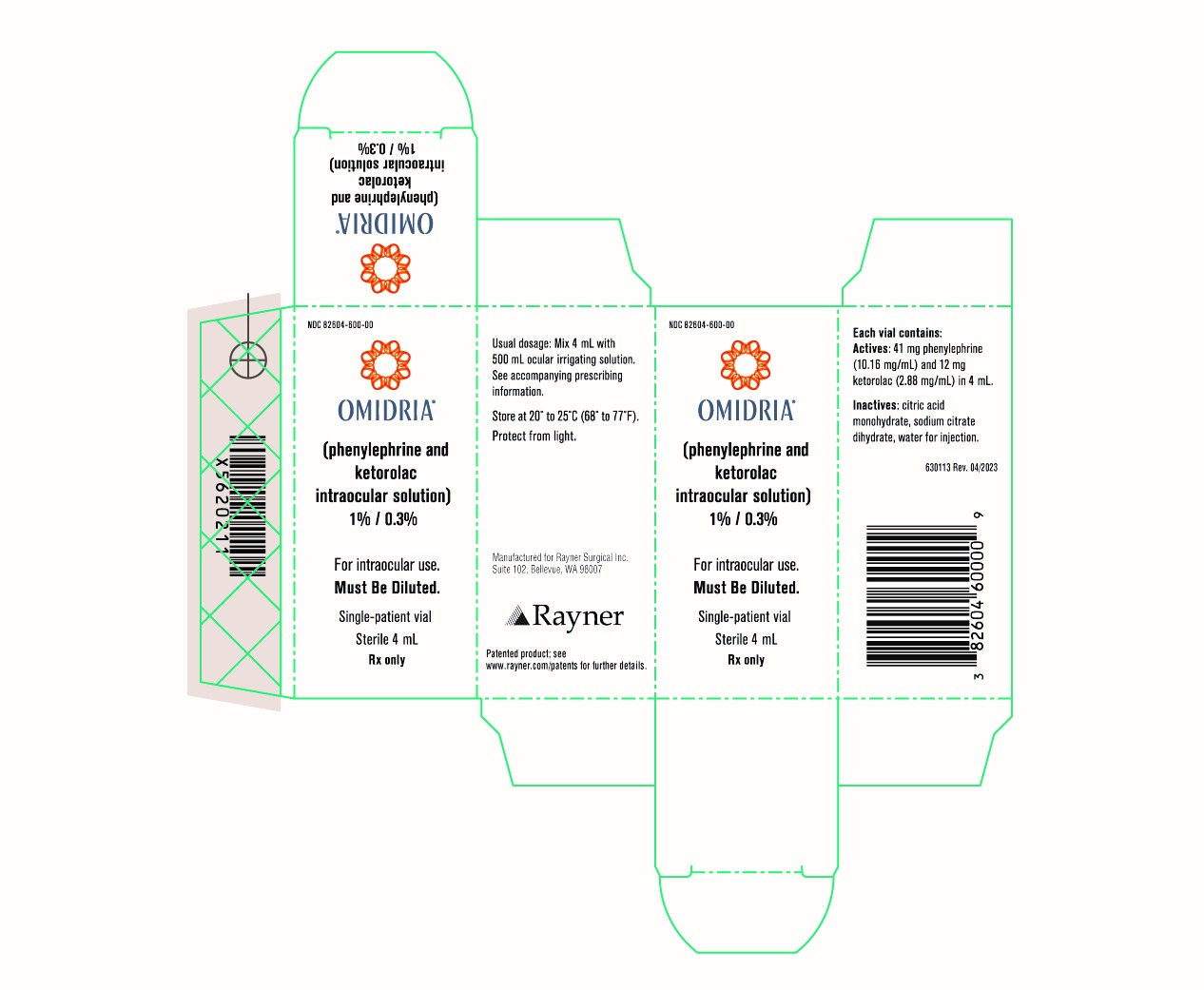
Omidria
What is Omidria (Ketorolac)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Opioids are commonly used after orthopedic surgery for pain control but have been shown to increase complications, surgeries, readmissions, and risk for opioid use disorder. The purpose of this study is to investigate the impact of adding a methylprednisolone taper to the pain regimen after ACL repair surgery to determine if this results in decreased postoperative pain and opioid use without incre...
Summary: Nationally, the opioid crisis has become a major epidemic with increasing mortality rates each year. Orthopedic surgeons routinely prescribe narcotics instead of NSAIDs for post-op pain control because of risk of delayed healing and nonunion due to NSAID use. Orthopedic oncology, however, has a unique subset of patients that undergo prophylactic placement of intramedullary femoral nails. Because n...
Summary: The goal of this randomized clinical trial is to learn if the use of a low-dose nonsteroidal anti-inflammatory drug (NSAID), ketorolac, reduces the rate of chronic opioid use in orthopaedic polytrauma patients. The main questions this study aims to answer are: 1. Are patients who are given scheduled ketorolac during the first five hospital days less likely to develop chronic opioid use at 6 months...
Related Latest Advances
Brand Information
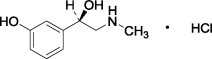
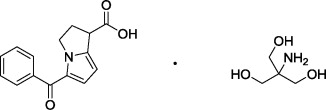
Patented product; see www.rayner.com/patents for further details.
640069