Generic Name
Bromfenac
Brand Names
Bromsite, Prolensa
FDA approval date: April 05, 2013
Classification: Nonsteroidal Anti-inflammatory Drug
Form: Solution
What is Bromsite (Bromfenac)?
Bromfenac ophthalmic solution.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
Comparing Efficacy of Bromfenac 0.09%, Nepafenac 0.3% and Diclofenac 0.1% in Patients After Cataract Surgery
Summary: The goal of this clinical trial is to learn which of the drugs bromfenac 0.09%, nepafenac 0.3% and diclofenac 0.1% has better efficacy in treatment and prevention of cystoid macular oedema after cataract surgery. The main questions it aims to answer are: Which of the drugs has better efficacy in macular oedema prevention? What medical problems do participants have when taking different drugs? Rese...
Related Latest Advances
Brand Information
BROMSITE (bromfenac)
1INDICATIONS AND USAGE
BromSite (bromfenac ophthalmic solution) 0.075% is indicated for the treatment of postoperative inflammation and prevention of ocular pain in patients undergoing cataract surgery.
2DOSAGE FORM AND STRENGTHS
Topical ophthalmic solution: bromfenac 0.075%.
3CONTRAINDICATIONS
None
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Slow or Delayed Healing [see Warnings and Precautions (5.1)]
- Potential for Cross-Sensitivity [see Warnings and Precautions (5.2)]
- Increased Bleeding Time of Ocular Tissue [see Warnings and Precautions (5.3)]
- Keratitis and Corneal Reactions[see Warnings and Precautions (5.4)]
- Contact Lens Wear [see Warnings and Precautions (5.5)]
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
5DESCRIPTION
BromSite (bromfenac ophthalmic solution) 0.075% is a sterile aqueous, topical NSAID, formulated in DuraSite
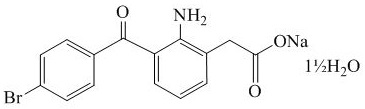
Bromfenac sodium is a bright orange to yellow powder. The molecular weight of bromfenac sodium sesquihydrate is 383.17. BromSite is a greenish-yellow to dark yellow viscous liquid with an osmolality of approximately 290 mOsmol/kg.
Active:Each mL contains bromfenac sodium sesquihydrate 0.87 mg, which is equivalent to bromfenac free acid 0.76 mg.
Preservative:benzalkonium chloride 0.005%
Inactives:boric acid, sodium borate, citric acid anhydrous, sodium citrate dihydrate, poloxamer 407, polycarbophil, sodium chloride, edetate disodium dihydrate, sodium hydroxide (to adjust pH to 8.3), and water for injection (USP).
6HOW SUPPLIED/STORAGE AND HANDLING
BromSite (bromfenac ophthalmic solution) 0.075% is supplied in white opaque low density polyethylene (LDPE) plastic bottles and translucent dropper tips, and gray high density polyethylene (HDPE) eyedropper caps. A white tamper evident overcap is provided. Each bottle is provided in a sealed foil laminated pouch.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Sterility of Dropper Tip/Product Use
Advise patients to replace the bottle cap after use and do not touch the dropper tip to any surface as this may contaminate the contents.
Advise patients to thoroughly wash hands prior to using BromSite.
Advise patients to replace the bottle cap after use and do not touch the dropper tip to any surface as this may contaminate the contents.
Advise patients to thoroughly wash hands prior to using BromSite.
Distributed by:
US Patent No. 8,778,999
BromSite and DuraSite are registered trademarks of Sun Pharmaceutical Industries Limited
© 2022 Sun Pharmaceutical Industries, Inc. All rights reserved.
Rev: MM/YYYY
VISX001-642R02
8INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
BromSite®[BRŏM- sahyt]
(bromfenac ophthalmic solution) 0.075%
BromSite®[BRŏM- sahyt]
(bromfenac ophthalmic solution) 0.075%
Read this Instructions for Use before you start using BromSite and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Information about BromSite:
•
Before you use BromSite for the first time:
• Tear open the foil pouch using the perforated notch and remove the BromSite bottle. Throw away the foil pouch.
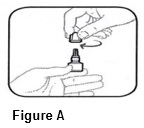
• Hold the bottle upright. Remove the gray cap by turning it in the counterclockwise direction (
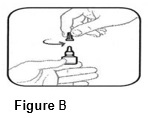
• Replace the gray cap on the bottle and close tightly.
Follow Steps 1 to 5 each time you use BromSite.
Step 1.Wash your hands well.
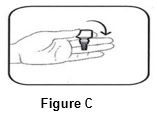
Step 2.Turn the closed bottle upside down ( See FIGURE C).
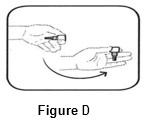
Step 3.Flick bottle firmly 1 time before each use to move the medicine into the tip of the bottle ( See FIGURE D).
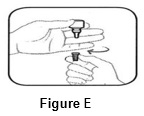
Step 4.Keep the bottle upside down and remove the gray cap by turning it in the clockwise direction ( See FIGURE E).
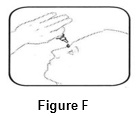
Step 5.Tilt your head back. Gently squeeze the bottle to place 1 drop into the affected eye ( See FIGURE F). Replace the gray cap on the bottle and close tightly.
How do I store BromSite?
• Store at 15°C to 25°C (59°F to 77°F). After opening, BromSite can be used until the expiration date on the bottle.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
BromSite and DuraSite are registered trademarks of Sun Pharmaceutical Industries Limited
Rev.: 03/2023
9PRINCIPAL DISPLAY PANEL - NDC: 49708-754-41 - 5 ML FOIL WRAPPER
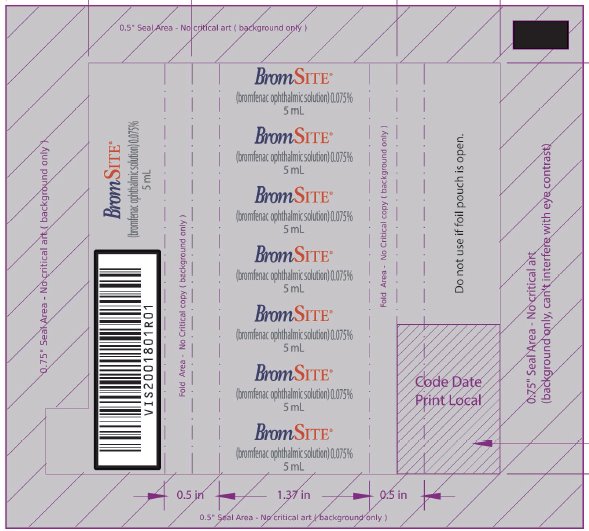
10PRINCIPAL DISPLAY PANEL - NDC: 49708-754-41 - 5 ML BOTTLE LABEL

11PRINCIPAL DISPLAY PANEL - NDC: 49708-754-41 - 5 ML CARTON LABEL

