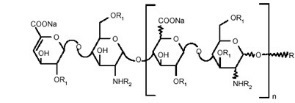
Enoxaparin
What is Lovenox (Enoxaparin)?
Related Clinical Trials
Summary: This study is researching an experimental drug called REGN7508 (called study drug). The study is focused on adults undergoing elective, unilateral (one side) total knee replacement surgery. The aim of the study is to see how effective the study drug is at preventing venous thromboembolism (VTE) and other related diseases after total knee replacement surgery. The study is looking at several other r...
Summary: The goal of this clinical trial is to learn if apixaban (a pill) is a safe and easier alternative to taking enoxaparin (a daily shot) to prevent blood clots after head and neck cancer surgery. It will also learn about side effects of both medicines. The main questions it aims to answer are: Can apixaban be used safely instead of enoxaparin to prevent blood clots after surgery? Do patients find api...
Summary: The goal of this observational study is to analyse the association between anti-factor Xa activity (antiXa) and the occurence of venous thromboembolism (VTE; either deep vein thrombosis and/or pulmonary embolism) in critically ill patients who are admitted to an intensive care unit. The main questions it aims to answer are: * What is the association between antiXa and VTE? * What is the associatio...
Related Latest Advances
Brand Information
- Use of indwelling epidural catheters
- Concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, and other anticoagulants
- A history of traumatic or repeated epidural or spinal punctures
- A history of spinal deformity or spinal surgery
- Optimal timing between the administration of Lovenox and neuraxial procedures is not known
- Active major bleeding
- History of immune-mediated heparin-induced thrombocytopenia (HIT) within the past 100 days or in the presence of circulating antibodies
- Known hypersensitivity to enoxaparin sodium (e.g., pruritus, urticaria, anaphylactic/anaphylactoid reactions)
- Known hypersensitivity to heparin or pork products
- Known hypersensitivity to benzyl alcohol (which is in only the multiple-dose formulation of Lovenox)
- Spinal/epidural hematomas
- Increased Risk of Hemorrhage
- Thrombocytopenia
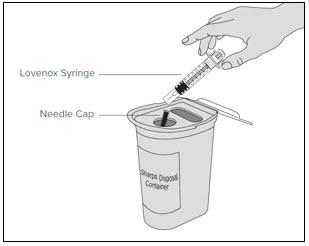
- of the instructions for injecting Lovenox if they continue Lovenox therapy after discharge from the hospital.
- that it may take them longer than usual to stop bleeding.
- that they may bruise and/or bleed more easily when they use Lovenox.
- that they should report any unusual bleeding, bruising, or signs of thrombocytopenia (such as a rash of dark red spots under the skin) to their physician
- that risks are associated with the use of benzyl alcohol, a preservative in Lovenox multiple-dose vials, in neonates, infants, and pregnant women.
- to tell their physicians and dentists they are taking Lovenox and/or any other product known to affect bleeding before any surgery is scheduled and before any new drug is taken
- to tell their physicians and dentists of all medications they are taking, including those obtained without a prescription, such as aspirin or other NSAIDs
(enoxaparin sodium injection)
for subcutaneous use
- Store Lovenox prefilled syringes at 77°F (25°C).
- Store Lovenox prefilled syringes in the original carton or packaging until ready to use.
- Keep Lovenox and all medicines out of the reach of children.
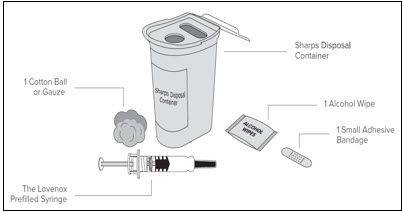
- 1 Lovenox prefilled syringe
- 1 alcohol wipe
- 1 cotton ball or gauze
- a small adhesive bandage, if needed
- a sharps disposal container
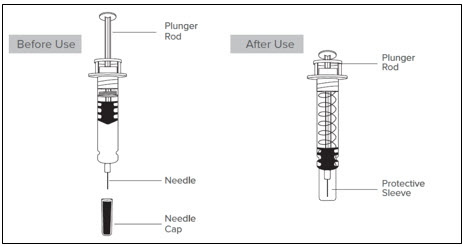
- Do not remove the prefilled syringe by pulling on the plunger rod or the needle cap as this may damage the syringe.
- Do not pull off the needle cap until you are ready to inject.
- Do not use the Lovenox prefilled syringe if it has been dropped on a hard surface or damaged.
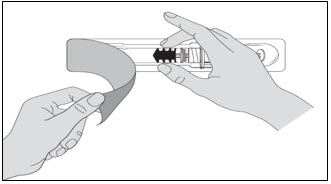
- When you receive your Lovenox syringes, always check to see that:
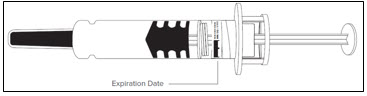
- Do not use the Lovenox prefilled syringe if the expiration date has passed.
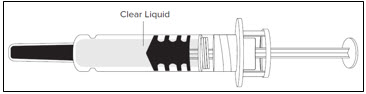
- Look at the medicine inside the Lovenox prefilled syringe:
- Do not use the Lovenox prefilled syringe if the liquid is discolored or cloudy, or if it contains visible flakes or particles.
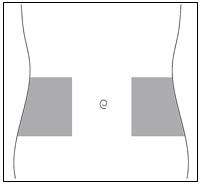
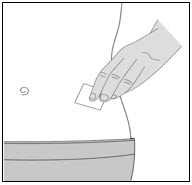
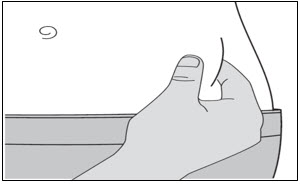
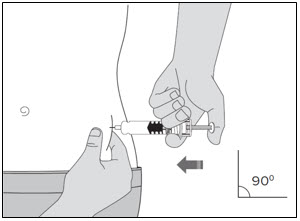
- You can inject into either the right or left side of your stomach area (abdomen), at least 2 inches away from your belly button and out towards your side (see
- You should alternate between the left or right side of your stomach each time you give yourself an injection.
- Do not inject into skin that has bruises or scars.
- Do not inject through clothes.
- Do not twist the needle cap to avoid bending the needle.
- Do not put the needle cap back on.
- Do not touch the needle.
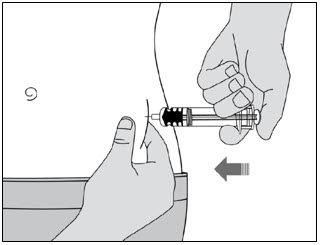
- Do not put the needle cap back on.
- Do not rub your skin after the injection.
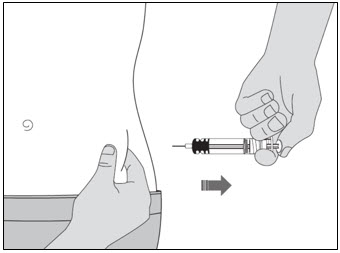
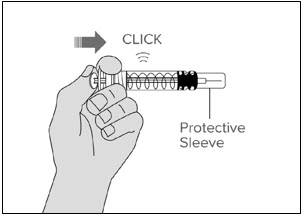
- You will feel some resistance. This is normal. Keep pushing until you hear the "click."
- The safety system can only be activated after the syringe has been emptied.
- Only activate the safety system after you have removed the needle from your skin.
- Activation of the safety system may cause a small amount of liquid to leak out of the syringe.
Activate the system while facing the syringe away from yourself and other people.
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.