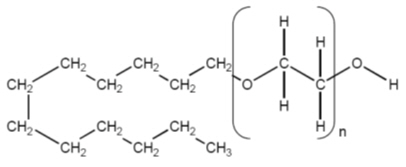
Polidocanol
What is Asclera (Polidocanol)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The aim of the study is to make a validation of Caprini score in patients undergoing varicose veins surgery, especially endovascular procedures (endovascular laser treatment - EVLT, radiofrequency ablation - RFA, ultrasound-guided foam sclerotherapy - USFS) and to identify patients with elevated risk of postoperative venous thromboembolism (VTE) who will benefit from prophylactic anticoagulation.
Summary: There is no consensus in the scientific literature for the treatment of aneurysmal and simple bone cysts. Some scientific articles with utilisation of sclerosis agents for the treatment of aneurysmal bone cysts: Ethibloc no longer marketed, pure Ethanol, Aetoxisclerol.
Summary: The current study aims to access the feasibility, safety, and efficacy of EUS-FNI for nonfunctional pNETs
Related Latest Advances
Brand Information
NDC 67850-140-05 Five 0.5 % ampules [10 mg/2 mL (5 mg/mL)]
NDC 67850-141-05 Five 1 % ampules [20 mg/2 mL (10 mg/mL)]
Each ampule is intended for immediate use in a single patient. Each unopened ampule is stable up to three years.
Chemische Fabrik Kreussler & Co. GmbH
(polidocanol) Injection
(10 mg per mL)

(polidocanol) Injection
(5 mg per mL)


