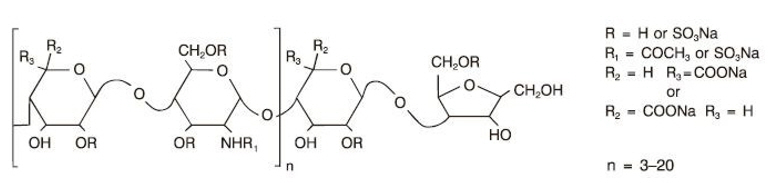
Fragmin
What is Fragmin (Dalteparin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Patients with cancer are prone to have blood clots, which are usually treated with blood thinners. The main complication of blood thinners is bleeding. This is especially a concern when the number of platelets in the blood is lower than 50,000 per microliter. The role of platelets is to stop bleeding, so when the number of platelets is low, patients are at a higher risk of bleeding. Cancer patient...
Summary: This is a Phase 3, multicenter, open-label, blinded endpoint study to evaluate the effect of abelacimab relative to dalteparin on venous thromboembolism (VTE) recurrence and bleeding in patients with gastrointestinal (GI)/genitourinary (GU) cancer associated VTE (Magnolia)
Summary: This is an international, open-label, stratified randomized controlled trial with Bayesian adaptive stopping rules to compare the effects of therapeutic-dose heparin vs. usual care pharmacological thromboprophylaxis on outcomes in patients admitted to hospital with community acquired pneumonia (CAP).
Related Latest Advances
Brand Information
- Use of indwelling epidural catheters
- Concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants.
- A history of traumatic or repeated epidural or spinal punctures
- A history of spinal deformity or spinal surgery
- Optimal timing between the administration of FRAGMIN and neuraxial procedures is not known
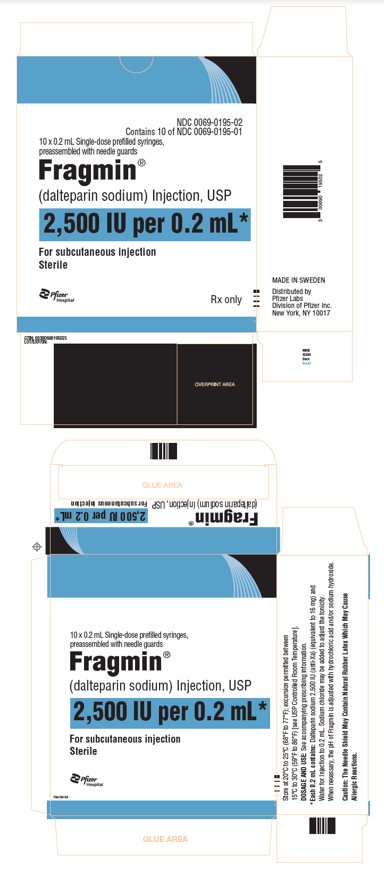
- Injection: 2,500 units/ 0.2 mL, 5,000 units/ 0.2 mL, 7,500 units/ 0.3 mL, 12,500 units/ 0.5 mL, 15,000 units/ 0.6 mL, and 18,000 units/ 0.72 mL sterile, single-dose, prefilled syringes preassembled with a needle guard device.
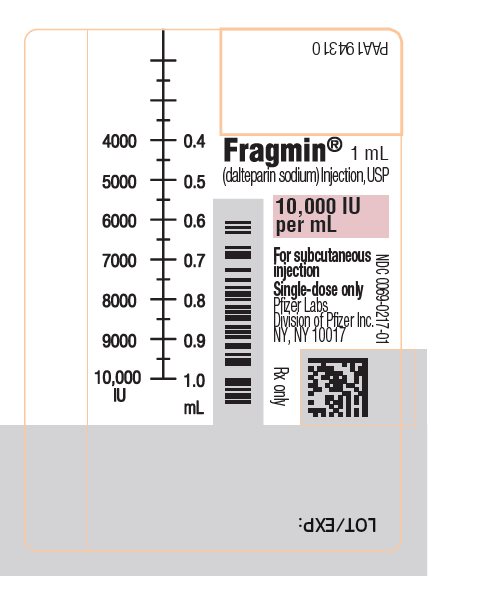
- Injection: 10,000 units/mL sterile, single-dose, graduated syringes preassembled with a needle guard device.
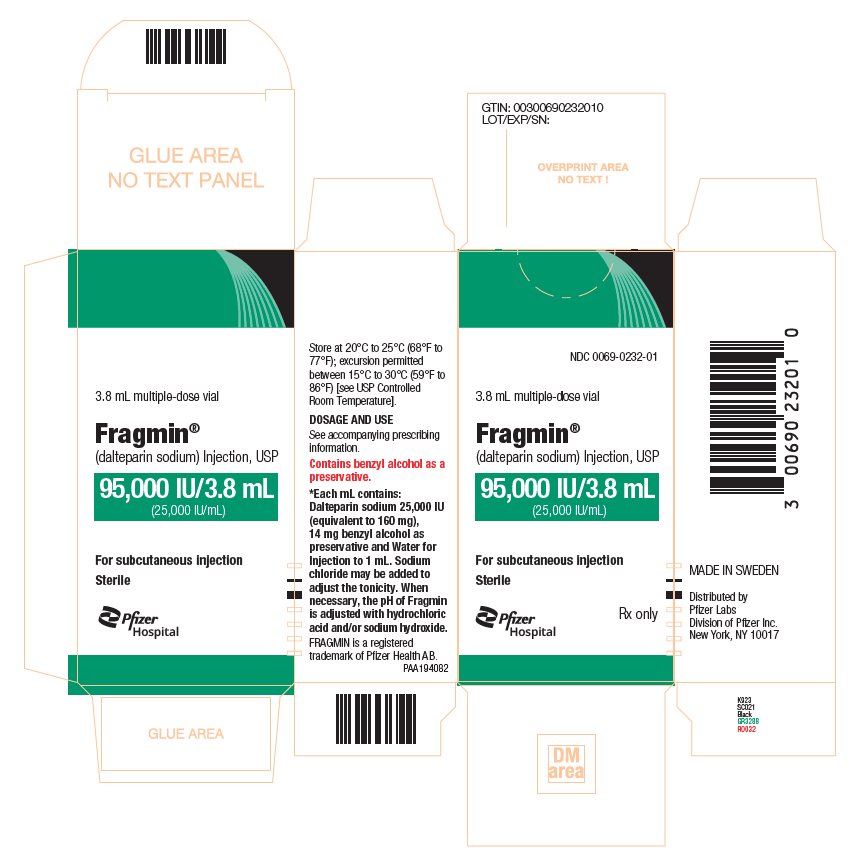
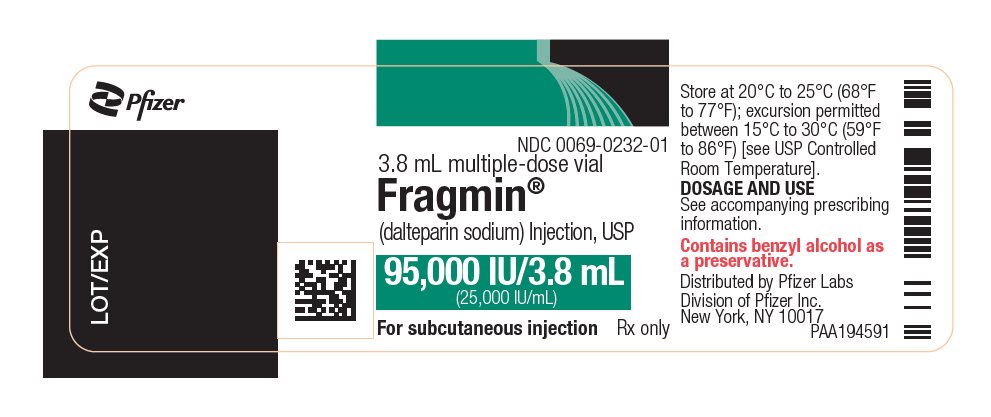
- Injection: 95,000 units/ 3.8 mL (25,000 units/mL) sterile, multiple-dose vials.
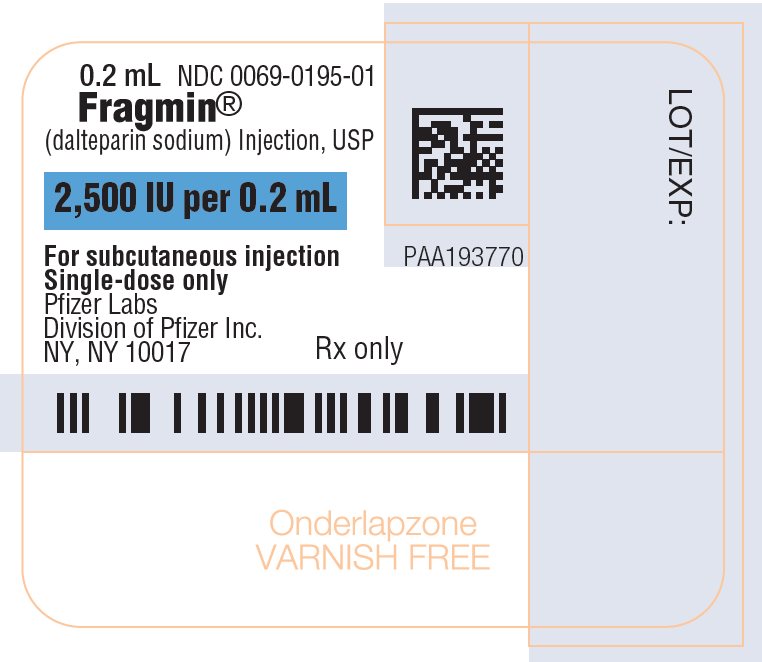
- Injection: 10,000 units/ 4 mL (2,500 units/mL) sterile, single-dose vials.
- Patients with active major bleeding.
- Patients with a history of heparin induced thrombocytopenia or heparin induced thrombocytopenia with thrombosis.
- Patients with prior hypersensitivity to dalteparin sodium (e.g., pruritis, rash, anaphylactic reactions)
- Patients undergoing Epidural/Neuraxial anesthesia, do not administer FRAGMIN
- Patients with prior hypersensitivity to heparin or pork products.
- Risk of Hemorrhage including Spinal/Epidural Hematomas
- Thrombocytopenia
- Benzyl Alcohol Preservative Risk to Premature Infants
(dalteparin sodium) Injection, USP
Single-dose prefilled syringe
Rx only
PAA233721

(dalteparin sodium) Injection, USP
Hospital
(dalteparin sodium) Injection, USP
Division of Pfizer Inc.
NY, NY 10001

Rx only
PAA233722

(dalteparin sodium) Injection, USP
SterilePreservative Free
Hospital

(dalteparin sodium) Injection, USP
Division of Pfizer Inc.
NY, NY 10001

Rx only
PAA233723

(dalteparin sodium) Injection, USP

(dalteparin sodium) Injection, USP
Pfizer Labs
Division of Pfizer Inc.
NY, NY 10001
Rx only
PAA233724

(dalteparin sodium) Injection, USP
Hospital

(dalteparin sodium) Injection, USP
Division of Pfizer Inc.
NY, NY 10001

Rx only
PAA233725

(dalteparin sodium) Injection, USP
SterilePreservative Free
Hospital

(dalteparin sodium) Injection, USP
Division of Pfizer Inc.
NY, NY 10001

Rx only
PAA233727

(dalteparin sodium) Injection, USP
Hospital

(dalteparin sodium) Injection, USP
Single-dose prefilled syringe

Rx only
PAA233726

(dalteparin sodium) Injection, USP
Hospital

(dalteparin sodium) Injection, USP
(25,000 units/mL)
Rx only
(dalteparin sodium) Injection, USP
(25,000 units/mL)
Sterile
Hospital