Generic Name
Phenytoin
Brand Names
Infatabs, Phenytoin Infatabs, Phenytek, Dilantin-125
FDA approval date: July 16, 1975
Classification: Anti-epileptic Agent
Form: Injection, Tablet, Suspension, Capsule
What is Infatabs (Phenytoin)?
Phenytoin is indicated for the treatment of tonic-clonic and psychomotor seizures. Phenytoin is indicated for the treatment of tonic-clonic and psychomotor seizures.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Dilantin Infatabs (Phenytoin)
1INDICATIONS AND USAGE
DILANTIN INFATABS are indicated for the treatment of generalized tonic-clonic (grand mal) and complex partial (psychomotor, temporal lobe) seizures and prevention and treatment of seizures occurring during or following neurosurgery.
2DOSAGE FORMS AND STRENGTHS
DILANTIN INFATABS are available as 50 mg phenytoin yellow triangular scored chewable tablets.
3CONTRAINDICATIONS
DILANTIN is contraindicated in patients with:
- A history of hypersensitivity to phenytoin, its inactive ingredients, or other hydantoins
- A history of prior acute hepatotoxicity attributable to phenytoin
- Coadministration with delavirdine because of the potential for loss of virologic response and possible resistance to delavirdine or to the class of non-nucleoside reverse transcriptase inhibitors.
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Withdrawal Precipitated Seizure, Status Epilepticus
- Suicidal Behavior and Ideation
- Serious Dermatologic Reactions
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
- Hypersensitivity
- Cardiac Effects
- Angioedema
- Hepatic Injury
- Hematopoietic Complications
- Effects on Vitamin D and Bone
- Exacerbation of Porphyria
- Teratogenicity and Other Harm to the Newborn
- Hyperglycemia
- The following adverse reactions associated with the use of DILANTIN were identified in clinical studies or postmarketing reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: Allergic reactions in the form of rash and rarely more serious forms and DRESS have been observed, as has angioedema [see . Anaphylaxis has also been reported.
There have also been reports of coarsening of facial features, systemic lupus erythematosus, periarteritis nodosa, and immunoglobulin abnormalities.
Digestive System: Acute hepatic failure, toxic hepatitis, liver damage, nausea, vomiting, constipation, enlargement of the lips, and gingival hyperplasia.
Hematologic and Lymphatic System: Hematopoietic complications, some fatal, have occasionally been reported in association with administration of phenytoin. These have included thrombocytopenia, leukopenia, granulocytopenia, agranulocytosis, and pancytopenia with or without bone marrow suppression. While macrocytosis and megaloblastic anemia have occurred, these conditions usually respond to folic acid therapy. Lymphadenopathy including benign lymph node hyperplasia, pseudolymphoma, lymphoma, and Hodgkin's disease have been reported [see . Pure red cell aplasia has also been reported.
Laboratory Test Abnormality: Phenytoin may decrease serum concentrations of thyroid hormone (T4 and T3), sometimes with an accompanying increase in thyroid-stimulating hormone (TSH), but usually in the absence of clinical hypothyroidism. Phenytoin may also produce lower than normal values for dexamethasone or metyrapone tests. Phenytoin may cause increased serum levels of glucose [see , alkaline phosphatase, and gamma glutamyl transpeptidase (GGT).
Nervous System: The most common adverse reactions encountered with phenytoin therapy are nervous system reactions and are usually dose-related. Reactions include nystagmus, ataxia, slurred speech, decreased coordination, somnolence, and mental confusion. Dizziness, vertigo, insomnia, transient nervousness, motor twitchings, paresthesias, and headaches have also been observed. There have also been rare reports of phenytoin-induced dyskinesias, including chorea, dystonia, tremor and asterixis, similar to those induced by phenothiazine and other neuroleptic drugs. Cerebellar atrophy has been reported, and appears more likely in settings of elevated phenytoin levels and/or long-term phenytoin use [see .
A predominantly sensory peripheral polyneuropathy has been observed in patients receiving long-term phenytoin therapy.
Skin and Appendages: Dermatological manifestations sometimes accompanied by fever have included scarlatiniform or morbilliform rashes. A morbilliform rash (measles-like) is the most common; other types of dermatitis are seen more rarely. Other more serious forms which may be fatal have included bullous, exfoliative or purpuric dermatitis, acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, and toxic epidermal necrolysis [see . There have also been reports of hypertrichosis and urticaria.
Special Senses: Altered taste sensation including metallic taste.
Urogenital: Peyronie's disease
5DRUG INTERACTIONS
Phenytoin is extensively bound to plasma proteins and is prone to competitive displacement. Phenytoin is primarily metabolized by the hepatic cytochrome P450 enzyme CYP2C9 and to a lesser extent by CYP2C19, and is particularly susceptible to inhibitory drug interactions because it is subject to saturable metabolism. Inhibition of metabolism may produce significant increases in circulating phenytoin concentrations and enhance the risk of drug toxicity. Monitoring of phenytoin serum levels is recommended when a drug interaction is suspected.
Phenytoin is a potent inducer of hepatic drug-metabolizing enzymes.
5.1Drugs that Affect Phenytoin Concentrations
Table 2 includes commonly occurring drug interactions that affect phenytoin concentrations. However, this list is not intended to be inclusive or comprehensive. Individual prescribing information from relevant drugs should be consulted.
The addition or withdrawal of these agents in patients on phenytoin therapy may require an adjustment of the phenytoin dose to achieve optimal clinical outcome
5.2Drugs Affected by Phenytoin
Table 3 includes commonly occurring drug interactions affected by phenytoin. However, this list is not intended to be inclusive or comprehensive. Individual drug package inserts should be consulted. The addition or withdrawal of phenytoin during concomitant therapy with these agents may require adjustment of the dose of these agents to achieve optimal clinical outcome.
5.3Hyperammonemia with Concomitant Use of Valproate
Concomitant administration of phenytoin and valproate has been associated with an increased risk of valproate-associated hyperammonemia. Patients treated concomitantly with these two drugs should be monitored for signs and symptoms of hyperammonemia.
5.4Drug Enteral Feeding/Nutritional Preparations Interaction
Literature reports suggest that patients who have received enteral feeding preparations and/or related nutritional supplements have lower than expected phenytoin serum levels. It is therefore suggested that phenytoin not be administered concomitantly with an enteral feeding preparation. More frequent serum phenytoin level monitoring may be necessary in these patients.
5.5Drug/Laboratory Test Interactions
Care should be taken when using immunoanalytical methods to measure serum phenytoin concentrations.
6OVERDOSAGE
The lethal dose in pediatric patients is not known. The lethal dose in adults is estimated to be 2 to 5 grams. The initial symptoms are nystagmus, ataxia, and dysarthria. Other signs are tremor, hyperreflexia, lethargy, slurred speech, blurred vision, nausea, and vomiting. The patient may become comatose and hypotensive. Bradycardia and cardiac arrest have been reported
There are marked variations among individuals with respect to phenytoin serum levels where toxicity may occur. Nystagmus, on lateral gaze, usually appears at 20 mcg/mL, ataxia at 30 mcg/mL; dysarthria and lethargy appear when the serum concentration is over 40 mcg/mL, but as high a concentration as 50 mcg/mL has been reported without evidence of toxicity. As much as 25 times the therapeutic dose has been taken to result in a serum concentration over 100 mcg/mL with complete recovery. Irreversible cerebellar dysfunction and atrophy have been reported.
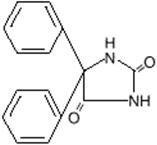
7DESCRIPTION
DILANTIN (phenytoin) is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is 5,5-diphenyl-2,4 imidazolidinedione, having the following structural formula:
Each DILANTIN INFATAB, chewable tablet for oral administration, contains 50 mg phenytoin, USP. Also contains: D&C yellow No. 10, Aluminum Lake; FD&C yellow No. 6, Aluminum Lake; flavor; saccharin sodium, USP; confectioner's sugar, NF; talc, USP; magnesium stearate, NF; and purified water, USP.
8PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Medication Guide).
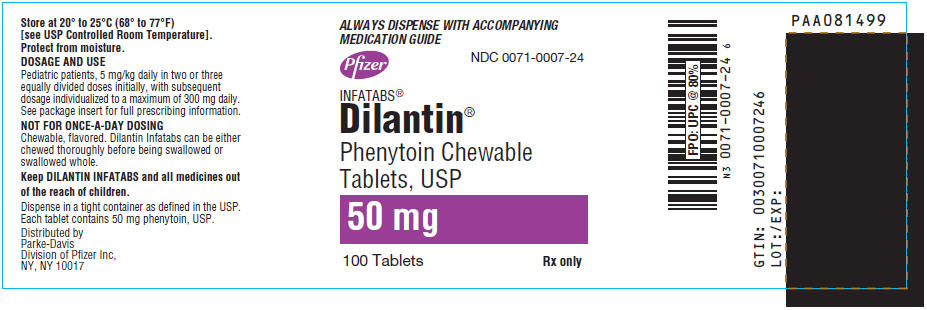
9PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label
ALWAYS DISPENSE WITH ACCOMPANYING
MEDICATION GUIDE
MEDICATION GUIDE
Pfizer
NDC 0071-0007-24
INFATABS
Phenytoin Chewable
50 mg
100 Tablets
10PRINCIPAL DISPLAY PANEL - 50 mg Tablet Blister Pack
INFATABS®
50 mg
Protect From Moisture.
DIST BY PARKE-DAVIS
EXP & LOT AREA

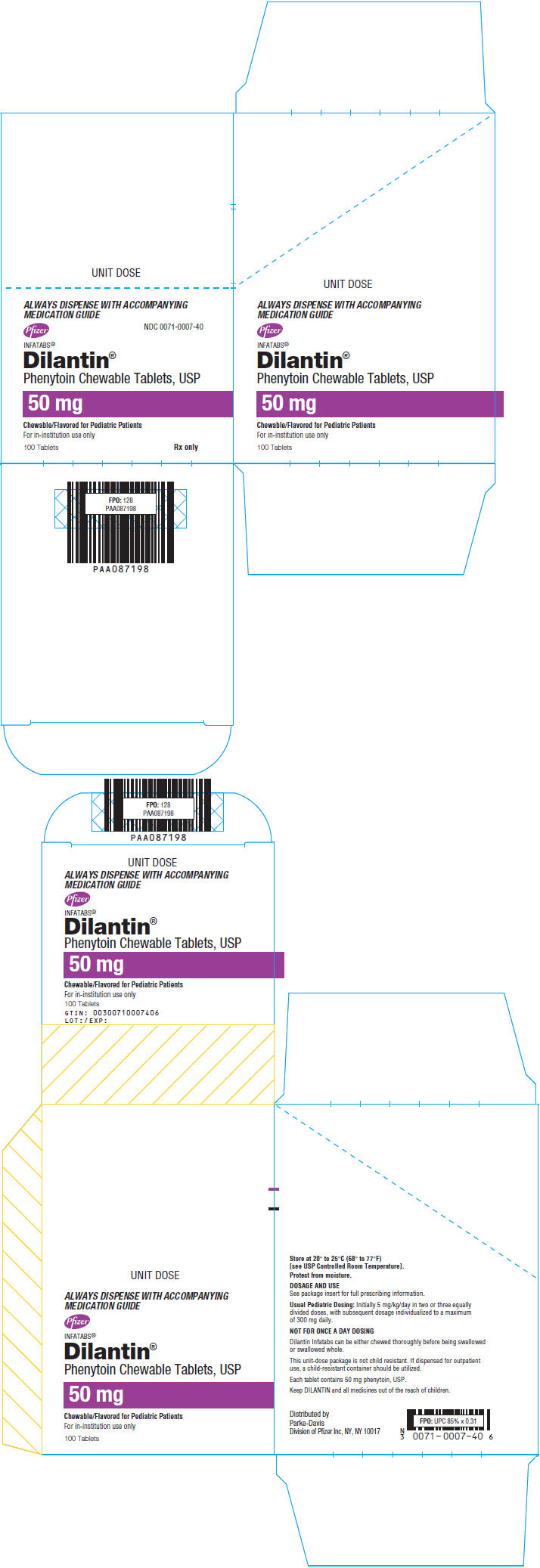
11PRINCIPAL DISPLAY PANEL - 50 mg Tablet Blister Pack Box
ALWAYS DISPENSE WITH ACCOMPANYING
MEDICATION GUIDE
MEDICATION GUIDE
Pfizer
NDC 0071-0007-40
INFATABS
Phenytoin Chewable Tablets, USP
50 mg
Chewable/Flavored for Pediatric Patients
For in-institution use only
100 Tablets