Ellence
What is Ellence (Epirubicin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Despite recent advances, the prognosis of patients with advanced gastric cancer remains poor. At present, regimens that combine a platinum and fluorouracil agent either alone or in combination with a third drug such as epirubicin or taxane constitute the most effective treatment option in the first-line metastatic setting, resulting in a median OS of approximately 10 months. In the second-line set...
Summary: Researchers are looking for new ways to treat types of breast cancer that are both: * High-risk, which means the cancer may have a higher chance of getting worse or coming back after treatment * Early-stage, which means the cancer is in the breast or the lymph nodes around the breast The 2 types of breast cancer in this study are triple-negative breast cancer (TNBC) and hormone receptor (HR)-low p...
Summary: This study is a multi-center, randomized, prospective phase II clinical trial aimed at exploring and evaluating the efficacy of dalpiciclib combined with AI in neoadjuvant treatment for ER strong positive(ER≥50%),HER2-negative, Ki-67≤20%,T1-3N1M0 postmenopausal breast cancer. The primary objectives are to demonstrate non-inferiority in efficacy compared to chemotherapy and to assess its superior s...
Related Latest Advances
Brand Information
- Cardiac Toxicity: Myocardial damage, including acute left ventricular failure, can occur with ELLENCE. The risk of cardiomyopathy is proportional to the cumulative exposure with incidence rates from 0.9% at a cumulative dose of 550 mg/m
- Secondary Malignancies: Secondary acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) occur at a higher incidence in patients treated with anthracyclines, including ELLENCE
- Extravasation and Tissue Necrosis: Extravasation of ELLENCE can result in severe local tissue injury and necrosis requiring wide excision of the affected area and skin grafting. Immediately terminate the drug and apply ice to the affected area
- Severe myelosuppression resulting in serious infection, septic shock, requirement for transfusions, hospitalization, and death may occur
- Severe myocardial insufficiency
- Recent myocardial infarction or severe arrhythmias, or previous treatment with maximum cumulative dose of anthracyclines
- Severe persistent drug-induced myelosuppression
- Severe hepatic impairment (defined as Child-Pugh Class C or serum bilirubin level greater than 5 mg/dL)
- Severe hypersensitivity to ELLENCE, other anthracyclines, or anthracenediones
- Cardiac Toxicity
- Secondary Malignancies
- Extravasation and Tissue Necrosis
- Severe Myelosuppression
- Tumor-Lysis Syndrome
- Thrombophlebitis and Thromboembolic Events
- Potentiation of Radiation Toxicity and Radiation Recall
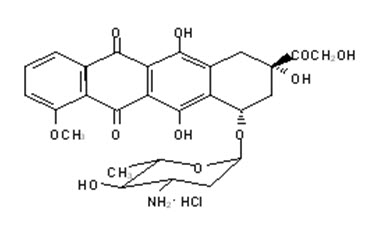
- "Hazardous Drugs".