Brand Name
Mayzent
Generic Name
Siponimod
View Brand Information FDA approval date: March 26, 2019
Classification: Sphingosine 1-phosphate Receptor Modulator
Form: Tablet
What is Mayzent (Siponimod)?
MAYZENT is indicated for the treatment of relapsing forms of multiple sclerosis , to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. MAYZENT is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of multiple sclerosis , to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Pregnancy Outcomes in Women Exposed to Diroximel Fumarate
Summary: The primary objective of the study is to estimate the prevalence of major congenital malformations (MCMs) and compare the prevalence between the diroximel fumarate (DRF) and comparator groups. The secondary objectives of the study are to estimate the incidence of spontaneous abortion (SA) and compare the incidence between the DRF and comparator groups; to estimate the incidence of preterm birth an...
Post-Authorization Safety Study for Assessment of Pregnancy Outcomes in Patients Treated With Mayzent (Siponimod): An OTIS Observational Pregnancy Surveillance Study
Summary: This study will utilize a prospective, observational, exposure cohort design to examine pregnancy and infant outcomes in women and infants who are exposed to siponimod during pregnancy to treat MS.
Related Latest Advances
Brand Information
MAYZENT (siponimod)
1INDICATIONS AND USAGE
MAYZENT is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
2DOSAGE FORMS AND STRENGTHS
0.25 mg tablet: Pale red, unscored, round biconvex film-coated tablet with beveled edges, debossed with
1 mg tablet: Violet white, unscored, round biconvex film-coated tablet with beveled edges, debossed with
2 mg tablet: Pale yellow, unscored, round biconvex film-coated tablet with beveled edges, debossed with
3CONTRAINDICATIONS
MAYZENT is contraindicated in patients who have:
- A CYP2C9*3/*3 genotype
- In the last 6 months experienced myocardial infarction, unstable angina, stroke, TIA, decompensated heart failure requiring hospitalization, or Class III or IV heart failure
- Presence of Mobitz type II second-degree, third-degree AV block, or sick sinus syndrome, unless patient has a functioning pacemaker
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Infections
- Progressive Multifocal Leukoencephalopathy
- Macular Edema
- Bradyarrhythmia and Atrioventricular Conduction Delays
- Respiratory Effects
- Liver Injury
- Cutaneous Malignancies
- Increased Blood Pressure
- Fetal Risk
- Posterior Reversible Encephalopathy Syndrome
- Unintended Additive Immunosuppressive Effects From Prior Treatment With Immunosuppressive or Immune-Modulating Therapies
- Severe Increase in Disability After Stopping MAYZENT
- Immune System Effects After Stopping MAYZENT
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 1737 MS patients have received MAYZENT at doses of at least 2 mg daily. These patients were included in Study 1
Table 3 lists adverse reactions that occurred in at least 5% of MAYZENT-treated patients and at a rate at least 1% higher than in patients receiving placebo.
The following adverse reactions have occurred in less than 5% of MAYZENT-treated patients but at a rate at least 1% higher than in patients receiving placebo: herpes zoster, lymphopenia, seizure, tremor, macular edema, AV block (1
Seizures
In Study 1, cases of seizures were reported in 1.7% of MAYZENT-treated patients, compared to 0.4% in patients receiving placebo. It is not known whether these events were related to the effects of MS, to MAYZENT, or to a combination of both.
Respiratory Effects
Dose-dependent reductions in forced expiratory volume over 1 second (FEV
Vascular Events
Vascular events, including ischemic strokes, pulmonary embolisms, and myocardial infarctions, were reported in 3.0% of MAYZENT-treated patients compared to 2.6% of patients receiving placebo. Some of these events were fatal. Physicians and patients should remain alert for the development of vascular events throughout treatment, even in the absence of previous vascular symptoms. Patients should be informed about the symptoms of cardiac or cerebral ischemia caused by vascular events and the steps to take if they occur.
Malignancies
Malignancies, such as BCC, SCC, malignant melanoma, and seminoma were reported in MAYZENT-treated patients in Study 1 (in the core or extension parts). An increased risk of cutaneous malignancies has been reported in association with S1P receptor modulators. The risk of BCC and SCC is increased in MAYZENT-treated patients
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of MAYZENT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections and Infestations: Progressive multifocal leukoencephalopathy [see Warnings and Precautions (5.2)].
5OVERDOSAGE
In patients with overdosage of MAYZENT, it is important to observe for signs and symptoms of bradycardia, which may include overnight monitoring. Regular measurements of pulse rate and blood pressure are required, and ECGs should be performed
There is no specific antidote to siponimod available. Neither dialysis nor plasma exchange would result in meaningful removal of siponimod from the body. The decrease in heart rate induced by MAYZENT can be reversed by atropine or isoprenaline.
6DESCRIPTION
MAYZENT tablets contains siponimod, an S1P receptor modulator, as 2:1 co-crystal of siponimod and fumaric acid and has the following chemical name:
1-[[4-[(1
Its structure is shown below:
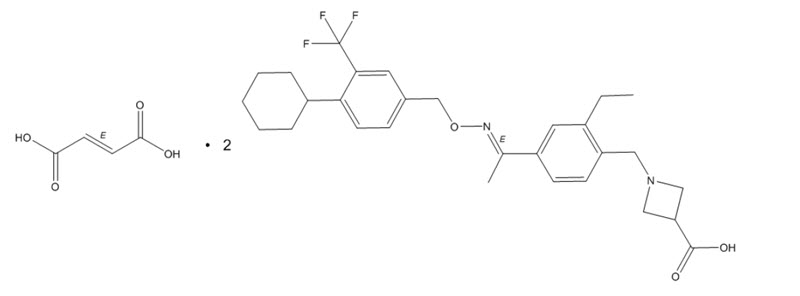
It is a white to almost white powder.
MAYZENT is provided as 0.25 mg, 1 mg, and 2 mg film-coated tablets for oral use. Each tablet contains 0.25 mg, 1 mg, or 2 mg siponimod, equivalent to 0.28 mg, 1.11 mg, or 2.22 mg as 2:1 co-crystal of siponimod and fumaric acid, respectively.
MAYZENT tablets contain the following inactive ingredients: colloidal silicon dioxide, crospovidone, glyceryl dibehenate, lactose monohydrate, microcrystalline cellulose, with a film coating containing iron oxides (black and red iron oxides for the 0.25 mg and 1 mg strengths and red and yellow iron oxides for the 2 mg strength), lecithin (soy), polyvinyl alcohol, talc, titanium dioxide, and xanthan gum.
7CLINICAL STUDIES
The efficacy of MAYZENT was demonstrated in Study 1, a randomized, double-blind, parallel-group, placebo-controlled, time-to-event study in patients with secondary progressive multiple sclerosis (SPMS) who had evidence of disability progression in the prior 2 years, no evidence of relapse in 3 months prior to study enrollment, and an Expanded Disability Status Scale (EDSS) score of 3.0 to 6.5 at study entry (NCT 01665144).
Patients were randomized to receive either once daily MAYZENT 2 mg or placebo, beginning with a dose titration
The primary endpoint of the study was the time to 3-month confirmed disability progression (CDP), defined as at least a 1-point increase from baseline in EDSS (0.5-point increase for patients with baseline EDSS of 5.5 or higher) sustained for 3 months. A prespecified hierarchical analysis consisted of the primary endpoint and 2 secondary endpoints, the time to 3-month confirmed worsening of at least 20% from baseline on the timed 25-foot walk test and the change from baseline in T2 lesion volume. Additional endpoints included annualized relapse rate (relapses/year) and MRI measures of inflammatory disease activity.
Study duration was variable for individual patients (median study duration was 21 months, range was 1 day to 37 months).
Study 1 randomized 1651 patients to either MAYZENT 2 mg (N = 1105) or placebo (N = 546); 82% of MAYZENT-treated patients and 78% of placebo-treated patients completed the study. Median age was 49.0 years, 95% of patients were white, and 60% female. The median disease duration was 16.0 years, and median EDSS score at baseline was 6.0 (56% of patients had ≥ 6.0 EDSS at baseline); 36% of patients had one or more relapses in the 2 years prior to study entry; 22% of those patients with available imaging had one or more gadolinium-enhancing lesions on their baseline MRI scan; 78% of patients had been previously treated with an MS therapy.
Results are presented in Table 4. MAYZENT was superior to placebo in reducing the risk of confirmed disability progression, based on a time-to-event analysis (hazard ratio 0.79,
Figure 1 Time to Confirmed Disability Progression Based on EDSS (Study 1)
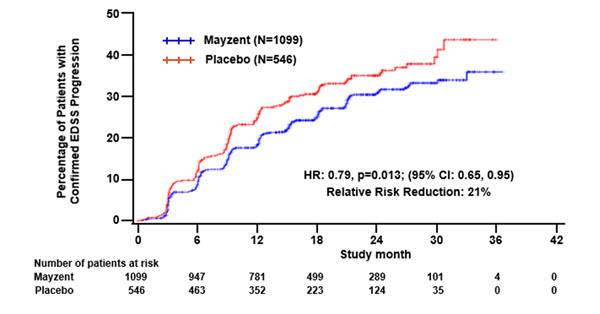
Figure 2 Time to Confirmed Disability Progression Based on EDSS (Study 1), Subgroup Analysis
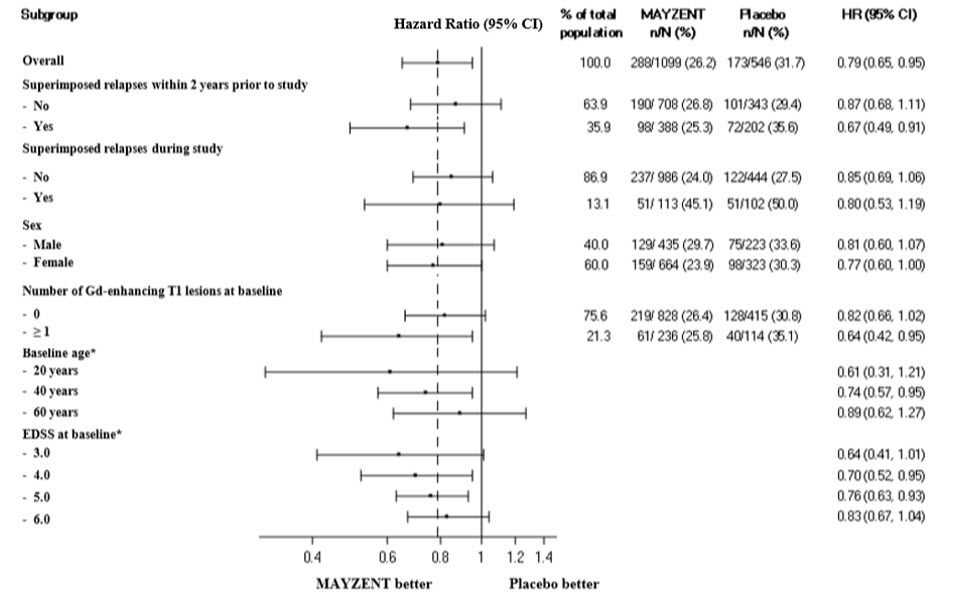
*HR and 95% CI presented are model-based estimates for a range of values of age and Expanded Disability Status Scale (EDSS).
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Administration
Tell patients not to discontinue MAYZENT without first discussing this with the prescribing physician. Advise patients to contact their physician if they accidently take more MAYZENT than prescribed.
Instruct patients to administer tablets whole; do not split, crush, or chew MAYZENT tablets.
Risk of Infections
Inform patients that they may have an increased risk of infections, some of which could be life-threatening, when taking MAYZENT, and that they should contact their physician if they develop symptoms of infection
Progressive Multifocal Leukoencephalopathy
Inform patients that cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients who received MAYZENT. Inform the patient that PML is characterized by a progression of deficits and usually leads to death or severe disability over weeks or months. Instruct the patient of the importance of contacting their doctor if they develop any symptoms suggestive of PML. Inform the patient that typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes
Macular Edema
Advise patients that MAYZENT may cause macular edema, and that they should obtain an eye exam near the start of treatment with MAYZENT, have their eyes monitored periodically by an eye care professional while receiving therapy, and contact their healthcare provider if they experience any changes in their vision while taking MAYZENT
Cardiac Effects
Advise patients that initiation of MAYZENT treatment results in transient decrease in heart rate
Respiratory Effects
Advise patients that they should contact their physician if they experience new onset or worsening of dyspnea
Liver Injury
Inform patients that MAYZENT may increase liver enzymes. Advise patients that they should contact their physician if they experience any unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine during treatment
Cutaneous Malignancies
Inform patients that the risk of BCC and SCC is increased with the use of MAYZENT and that cases of melanoma have been reported. Advise patients that any suspicious skin lesions should be promptly evaluated. Advise patients to limit exposure to sunlight and ultraviolet light by wearing protective clothing and using a sunscreen with a high protection factor
Pregnancy and Fetal Risk
Inform patients that, based on animal studies, MAYZENT may cause fetal harm
Pregnancy Exposure Registry
Instruct patients that if they are pregnant or plan to become pregnant while taking MAYZENT, they should inform their healthcare provider. Encourage patients to enroll in the MotherToBaby Pregnancy Study in Multiple Sclerosis if they become pregnant while taking MAYZENT
Posterior Reversible Encephalopathy Syndrome
Advise patients to immediately report to their healthcare provider any symptoms involving sudden onset of severe headache, altered mental status, visual disturbances, or seizure. Inform patients that delayed treatment could lead to permanent neurological sequelae
Severe Increase in Disability After Stopping MAYZENT
Inform patients that severe increase in disability has been reported after discontinuation of another S1P receptor modulator like MAYZENT. Advise patients to contact their physician if they develop worsening symptoms of MS following discontinuation of MAYZENT
Immune System Effects After Stopping MAYZENT
Advise patients that MAYZENT continues to have effects, such as lowering effects on peripheral lymphocyte count, for up to 3 to 4 weeks after the last dose
Storage and Handling
Inform patients that MAYZENT may be stored at room temperature for up to 3 months. If patients need to store MAYZENT for more than 3 months, containers should remain unopened and stored in a refrigerator until use
Distributed by:
MAYZENT is a registered trademark of Novartis AG
© Novartis
T2025-51
9PRINCIPAL DISPLAY PANEL
NDC 0078-0979-50
Rx only
MAYZENT
(siponimod) tablets
0.25 mg*
28 tablets
Dispense with accompanying Medication Guide.
NOVARTIS

10PRINCIPAL DISPLAY PANEL
NDC 0078-1014-15
Rx only
MAYZENT
(siponimod) tablets
1 mg*
30 tablets
Dispense with accompanying Medication Guide.
Swallow tablets whole. DO NOT split tablets.
NOVARTIS
11PRINCIPAL DISPLAY PANEL
NDC 0078-0986-15
Rx only
MAYZENT
(siponimod) tablets
2 mg*
30 tablets
Dispense with accompanying Medication Guide.
Swallow tablets whole. DO NOT split tablets.
NOVARTIS

