Generic Name
Imatinib
Brand Names
Gleevec, Imkeldi
FDA approval date: May 15, 2001
Classification: Kinase Inhibitor
Form: Tablet, Solution
What is Gleevec (Imatinib)?
Imatinib mesylate tablets are a kinase inhibitor indicated for the treatment of: Newly diagnosed adult and pediatric patients with Philadelphia chromosome positive chronic myeloid leukemia in chronic phase.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Gleevec (imatinib mesylate)
1DOSAGE FORMS AND STRENGTHS
- 100 mg film coated tablets
Very dark yellow to brownish orange, film-coated tablets, round, biconvex with bevelled edges, debossed with “NVR” on one side, and “SA” with score on the other side
- 400 mg film coated tablets
Very dark yellow to brownish orange, film-coated tablets, ovaloid, biconvex with bevelled edges, debossed with “gleevec” on one side and score on the other side.
Very dark yellow to brownish orange, film-coated tablets, ovaloid, biconvex with bevelled edges, debossed with “400” on one side and score on the other side with “SL” on each side of the score.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Fluid Retention and Edema
- Hematologic Toxicity
- Congestive Heart Failure and Left Ventricular Dysfunction
- Hepatotoxicity
- Hemorrhage
- Gastrointestinal Disorders
- Hypereosinophilic Cardiac Toxicity
- Dermatologic Toxicities
- Hypothyroidism
- Growth Retardation in Children and Adolescents
- Tumor Lysis Syndrome
- Impairments Related to Driving and Using Machinery
- Renal Toxicity
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Chronic Myeloid Leukemia
The majority of Gleevec-treated patients experienced adverse reactions at some time. Gleevec was discontinued due to drug-related adverse reactions in 2.4% of patients receiving Gleevec in the randomized trial of newly diagnosed patients with Ph+ CML in chronic phase comparing Gleevec versus IFN+Ara-C, and in 12.5% of patients receiving Gleevec in the randomized trial of newly diagnosed patients with Ph+ CML in chronic phase comparing Gleevec and nilotinib. Gleevec was discontinued due to drug-related adverse reactions in 4% of patients in chronic phase after failure of interferon-alpha therapy, in 4% of patients in accelerated phase and in 5% of patients in blast crisis.
The most frequently reported drug-related adverse reactions were edema, nausea and vomiting, muscle cramps, musculoskeletal pain, diarrhea and rash (Table 2 and Table 3 for newly diagnosed CML, Table 4 for other CML patients). Edema was most frequently periorbital or in lower limbs and was managed with diuretics, other supportive measures, or by reducing the dose of Gleevec
A variety of adverse reactions represent local or general fluid retention, including pleural effusion, ascites, pulmonary edema, and rapid weight gain with or without superficial edema. These reactions appear to be dose related, were more common in the blast crisis and accelerated phase studies (where the dose was 600 mg/day), and are more common in the elderly. These reactions were usually managed by interrupting Gleevec treatment and using diuretics or other appropriate supportive care measures. These reactions may be serious or life threatening.
Adverse reactions, regardless of relationship to study drug, that were reported in at least 10% of the Gleevec-treated patients are shown in Tables 2, 3, and 4.
Hematologic and Biochemistry Laboratory Abnormalities
Cytopenias, and particularly neutropenia and thrombocytopenia, were a consistent finding in all studies, with a higher frequency at doses greater than or equal to 750 mg (Phase 1 study). The occurrence of cytopenias in CML patients was also dependent on the stage of the disease.
In patients with newly diagnosed CML, cytopenias were less frequent than in the other CML patients (see Tables 5, 6, and 7). The frequency of Grade 3 or 4 neutropenia and thrombocytopenia was between 2- and 3-fold higher in blast crisis and accelerated phase compared to chronic phase (see Tables 4 and 5). The median duration of the neutropenic and thrombocytopenic episodes varied from 2 to 3 weeks, and from 2 to 4 weeks, respectively.
These reactions can usually be managed with either a reduction of the dose or an interruption of treatment with Gleevec, but may require permanent discontinuation of treatment.
Hepatotoxicity
Severe elevation of transaminases or bilirubin occurred in approximately 5% of CML patients (see Tables 6 and 7) and were usually managed with dose reduction or interruption (the median duration of these episodes was approximately 1 week). Treatment was discontinued permanently because of liver laboratory abnormalities in less than 1.0% of CML patients. One patient, who was taking acetaminophen regularly for fever, died of acute liver failure. In the Phase 2 GIST trial, Grade 3 or 4 SGPT (ALT) elevations were observed in 6.8% of patients and Grade 3 or 4 SGOT (AST) elevations were observed in 4.8% of patients. Bilirubin elevation was observed in 2.7% of patients.
Adverse Reactions in Pediatric Population
Single-Agent Therapy
The overall safety profile of pediatric patients treated with Gleevec in 93 children studied was similar to that found in studies with adult patients, except that musculoskeletal pain was less frequent (20.5%) and peripheral edema was not reported. Nausea and vomiting were the most commonly reported individual adverse reactions with an incidence similar to that seen in adult patients. Most patients experienced adverse reactions at some time during the study. The incidence of Grade 3/4 events across all types of adverse reactions was 75%; the events with the highest Grade 3/4 incidence in CML pediatric patients were mainly related to myelosuppression.
In Combination with Multi-Agent Chemotherapy
Pediatric and young adult patients with very high risk ALL, defined as those with an expected 5 year event-free survival (EFS) less than 45%, were enrolled after induction therapy on a multicenter, non-randomized cooperative group pilot protocol. The study population included patients with a median age of 10 years (1 to 21 years), 61% of whom were male, 75% were white, 7% were black, and 6% were Asian/Pacific Islander. Patients with Ph+ ALL (n = 92) were assigned to receive Gleevec and treated in 5 successive cohorts. Gleevec exposure was systematically increased in successive cohorts by earlier introduction and more prolonged duration.
The safety of Gleevec given in combination with intensive chemotherapy was evaluated by comparing the incidence of Grade 3 and 4 adverse events, neutropenia (less than 750/mcL) and thrombocytopenia (less than 75,000/mcL) in the 92 patients with Ph+ ALL compared to 65 patients with Ph- ALL enrolled on the trial who did not receive Gleevec. The safety was also evaluated comparing the incidence of adverse events in cycles of therapy administered with or without Gleevec. The protocol included up to 18 cycles of therapy. Patients were exposed to a cumulative total of 1425 cycles of therapy, 778 with Gleevec, and 647 without Gleevec. The adverse events that were reported with a 5% or greater incidence in patients with Ph+ ALL compared to Ph- ALL or with a 1% or greater incidence in cycles of therapy that included Gleevec are presented in Table 8.
Adverse Reactions in Other Subpopulations
In older patients (greater than or equal to 65 years old), with the exception of edema, where it was more frequent, there was no evidence of an increase in the incidence or severity of adverse reactions. In women there was an increase in the frequency of neutropenia, as well as Grade 1/2 superficial edema, headache, nausea, rigors, vomiting, rash, and fatigue. No differences were seen that were related to race but the subsets were too small for proper evaluation.
Acute Lymphoblastic Leukemia
The adverse reactions were similar for Ph+ ALL as for Ph+ CML. The most frequently reported drug-related adverse reactions reported in the Ph+ ALL studies were mild nausea and vomiting, diarrhea, myalgia, muscle cramps, and rash. Superficial edema was a common finding in all studies and were described primarily as periorbital or lower limb edemas. These edemas were reported as Grade 3/4 events in 6.3% of the patients and may be managed with diuretics, other supportive measures, or in some patients by reducing the dose of Gleevec.
Myelodysplastic/Myeloproliferative Diseases
Adverse reactions, regardless of relationship to study drug, that were reported in at least 10% of the patients treated with Gleevec for MDS/MPD in the Phase 2 study, are shown in Table 9.
Aggressive Systemic Mastocytosis
All aggressive systemic mastocytosis (ASM) patients experienced at least one adverse reaction at some time. The most frequently reported adverse reactions were diarrhea, nausea, ascites, muscle cramps, dyspnea, fatigue, peripheral edema, anemia, pruritus, rash, and lower respiratory tract infection. None of the 5 patients in the Phase 2 study with ASM discontinued Gleevec due to drug-related adverse reactions or abnormal laboratory values.
Hypereosinophilic Syndrome and Chronic Eosinophilic Leukemia
The safety profile in the HES/CEL patient population does not appear to be different from the safety profile of Gleevec observed in other hematologic malignancy populations, such as Ph+ CML. All patients experienced at least one adverse reaction, the most common being GI, cutaneous and musculoskeletal disorders. Hematological abnormalities were also frequent, with instances of CTC Grade 3 leukopenia, neutropenia, lymphopenia, and anemia.
Dermatofibrosarcoma Protuberans
Adverse reactions, regardless of relationship to study drug, that were reported in at least 10% of the 12 patients treated with Gleevec for DFSP in the Phase 2 study are shown in Table 10.
Clinically relevant or severe laboratory abnormalities in the 12 patients treated with Gleevec for DFSP in the Phase 2 study are presented in Table 11.
Gastrointestinal Stromal Tumors
Unresectable and/or Malignant Metastatic GIST
In the Phase 3 trials, the majority of Gleevec-treated patients experienced adverse reactions at some time. The most frequently reported adverse reactions were edema, fatigue, nausea, abdominal pain, diarrhea, rash, vomiting, myalgia, anemia, and anorexia. Drug was discontinued for adverse reactions in a total of 89 patients (5.4%). Superficial edema, most frequently periorbital or lower extremity edema was managed with diuretics, other supportive measures, or by reducing the dose of Gleevec
Adverse reactions, regardless of relationship to study drug, that were reported in at least 10% of the patients treated with Gleevec are shown in Table 12.
Overall the incidence of all grades of adverse reactions and the incidence of severe adverse reactions (CTC Grade 3 and above) were similar between the two treatment arms except for edema, which was reported more frequently in the 800 mg group.
Clinically relevant or severe abnormalities of routine hematologic or biochemistry laboratory values were not reported or evaluated in the Phase 3 GIST trials. Severe abnormal laboratory values reported in the Phase 2 GIST trial are presented in Table 13.
Adjuvant Treatment of GIST
In Study 1, the majority of both Gleevec and placebo-treated patients experienced at least one adverse reaction at some time. The most frequently reported adverse reactions were similar to those reported in other clinical studies in other patient populations and include diarrhea, fatigue, nausea, edema, decreased hemoglobin, rash, vomiting, and abdominal pain. No new adverse reactions were reported in the adjuvant GIST-treatment setting that had not been previously reported in other patient populations, including patients with unresectable and/or malignant metastatic GIST. Drug was discontinued for adverse reactions in 57 patients (17%) and 11 patients (3%) of the Gleevec and placebo-treated patients, respectively. Edema, GI disturbances (nausea, vomiting, abdominal distention, and diarrhea), fatigue, low hemoglobin, and rash were the most frequently reported adverse reactions at the time of discontinuation.
In Study 2, discontinuation of therapy due to adverse reactions occurred in 15 patients (8%) and 27 patients (14%) of the Gleevec 12-month, and 36-month treatment arms, respectively. As in previous trials the most common adverse reactions were diarrhea, fatigue, nausea, edema, decreased hemoglobin, rash, vomiting, and abdominal pain.
Adverse reactions, regardless of relationship to study drug, that were reported in at least 5% of the patients treated with Gleevec are shown in Table 14 (Study 1) and Table 15 (Study 2). There were no deaths attributable to Gleevec treatment in either trial.
Adverse Reactions from Multiple Clinical Trials
Cardiac Disorders:
Estimated 1%-10%: palpitations, pericardial effusion
Estimated 0.1%-1%: congestive cardiac failure, tachycardia, pulmonary edema
Estimated 0.01%-0.1%: arrhythmia, atrial fibrillation, cardiac arrest, myocardial infarction, angina pectoris
Vascular Disorders:
Estimated 1%-10%: flushing, hemorrhage
Estimated 0.1%-1%: hypertension, hypotension, peripheral coldness, Raynaud’s phenomenon, hematoma, subdural hematoma
Investigations:
Estimated 1%-10%: blood creatine phosphokinase (CPK) increased, blood amylase increased
Estimated 0.1%-1%: blood lactate dehydrogenase (LDH) increased
Skin and Subcutaneous Tissue Disorders:
Estimated 1%-10%: dry skin, alopecia, face edema, erythema, photosensitivity reaction, nail disorder, purpura
Estimated 0.1%-1%: exfoliative dermatitis, bullous eruption, psoriasis, rash pustular, contusion, sweating increased, urticaria, ecchymosis, increased tendency to bruise, hypotrichosis, skin hypopigmentation, skin hyperpigmentation, onychoclasis, folliculitis, petechiae, erythema multiforme, panniculitis (including erythema nodosum)
Estimated 0.01%-0.1%: vesicular rash, Stevens-Johnson syndrome, acute generalized exanthematous pustulosis, acute febrile neutrophilic dermatosis (Sweet’s syndrome), nail discoloration, angioneurotic edema, leucocytoclastic vasculitis
Gastrointestinal Disorders:
Estimated 1%-10%: abdominal distention, gastroesophageal reflux, dry mouth, gastritis
Estimated 0.1%-1%: gastric ulcer, stomatitis, mouth ulceration, eructation, melena, esophagitis, ascites, hematemesis, chelitis, dysphagia, pancreatitis
Estimated 0.01%-0.1%: colitis, ileus, inflammatory bowel disease
General Disorders and Administration-Site Conditions:
Estimated 1%-10%: weakness, anasarca, chills
Estimated 0.1%-1%: malaise
Blood and Lymphatic System Disorders:
Estimated 1%-10%: pancytopenia, febrile neutropenia, lymphopenia, eosinophilia
Estimated 0.1%-1%: thrombocythemia, bone marrow depression, lymphadenopathy
Estimated 0.01%-0.1%: hemolytic anemia, aplastic anemia
Hepatobiliary Disorders:
Estimated 0.1%-1%: hepatitis, jaundice
Estimated 0.01%-0.1%: hepatic failure and hepatic necrosis
Immune System Disorders:
Estimated 0.01%-0.1%: angioedema
Infections and Infestations:
Estimated 0.1%-1%: sepsis, herpes simplex, herpes zoster, cellulitis, urinary tract infection, gastroenteritis
Estimated 0.01%-0.1%: fungal infection
Metabolism and Nutrition Disorders:
Estimated 1%-10%: weight decreased, decreased appetite
Estimated 0.1%-1%: dehydration, gout, increased appetite, hyperuricemia, hypercalcemia, hyperglycemia, hyponatremia, hyperkalemia, hypomagnesemia
Musculoskeletal and Connective Tissue Disorders:
Estimated 1%-10%: joint swelling
Estimated 0.1%-1%: joint and muscle stiffness, muscular weakness, arthritis
Nervous System/Psychiatric Disorders:
Estimated 1%-10%: paresthesia, hypesthesia
Estimated 0.1%-1%: syncope, peripheral neuropathy, somnolence, migraine, memory impairment, libido decreased, sciatica, restless leg syndrome, tremor
Estimated 0.01%-0.1%: increased intracranial pressure
Renal and Urinary Disorders:
Estimated 0.1%-1%: renal failure acute, urinary frequency increased, hematuria, renal pain
Reproductive System and Breast Disorders:
Estimated 0.1%-1%: breast enlargement, menorrhagia, sexual dysfunction, gynecomastia, erectile dysfunction, menstruation irregular, nipple pain, scrotal edema
Respiratory, Thoracic and Mediastinal Disorders:
Estimated 1%-10%: epistaxis
Estimated 0.1%-1%: pleural effusion
Estimated 0.01%-0.1%: interstitial pneumonitis, pulmonary fibrosis, pleuritic pain, pulmonary hypertension, pulmonary hemorrhage
Endocrine Disorders:
Estimated 0.1%-1%: hypothyroidism, hyperthyroidism
Eye, Ear, and Labyrinth Disorders:
Estimated 1%-10%: conjunctivitis, vision blurred, orbital edema, conjunctival hemorrhage, dry eye
Estimated 0.1%-1%: vertigo, tinnitus, eye irritation, eye pain, scleral hemorrhage, retinal hemorrhage, blepharitis, macular edema, hearing loss, cataract
Estimated 0.01%-0.1%: papilledema
1Including some fatalities.
3.2Postmarketing Experience
The following additional adverse reactions have been identified during post approval use of Gleevec. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: thrombotic microangiopathy
Cardiac Disorders: pericarditis, cardiac tamponade1
Eye Disorders: vitreous hemorrhage
Gastrointestinal Disorders: ileus/intestinal obstruction, tumor hemorrhage/tumor necrosis, GI perforation1 [see Warnings and Precautions (5.6)], diverticulitis, gastric antral vascular ectasia
Infections: hepatitis B virus reactivation1
Musculoskeletal and Connective Tissue Disorders: osteonecrosis, rhabdomyolysis/myopathy, growth retardation in children, musculoskeletal pain upon treatment discontinuation (including myalgia, pain in extremity, arthalgia, bone pain)
Nervous System Disorders: cerebral edema1
Reproduction Disorders: hemorrhagic corpus luteum/hemorrhagic ovarian cyst
Respiratory, Thoracic and Mediastinal Disorders: acute respiratory failure1, interstitial lung disease
Skin and Subcutaneous Tissue Disorders: lichenoid keratosis, lichen planus, toxic epidermal necrolysis, palmar-plantar erythrodysesthesia syndrome, drug rash with eosinophilia and systemic symptoms (DRESS), pseudoporphyria, pemphigus
Vascular Disorders: thrombosis/embolism, anaphylactic shock
1Including some fatalities.
4OVERDOSAGE
Experience with doses greater than 800 mg is limited. Isolated cases of Gleevec overdose have been reported. In the event of overdosage, observe the patient and give appropriate supportive treatment.
Adult Overdose
1,200 to 1,600 mg (duration varying between 1 to 10 days): Nausea, vomiting, diarrhea, rash erythema, edema, swelling, fatigue, muscle spasms, thrombocytopenia, pancytopenia, abdominal pain, headache, decreased appetite.
1,800 to 3,200 mg (as high as 3,200 mg daily for 6 days): Weakness, myalgia, increased CPK, increased bilirubin, GI pain.
6,400 mg (single dose): One case in the literature reported one patient who experienced nausea, vomiting, abdominal pain, pyrexia, facial swelling, neutrophil count decreased, increase transaminases.
8 to 10 g (single dose): Vomiting and GI pain have been reported.
A patient with myeloid blast crisis experienced Grade 1 elevations of serum creatinine, Grade 2 ascites and elevated liver transaminase levels, and Grade 3 elevations of bilirubin after inadvertently taking 1,200 mg of Gleevec daily for 6 days. Therapy was temporarily interrupted and complete reversal of all abnormalities occurred within 1 week. Treatment was resumed at a dose of 400 mg daily without recurrence of adverse reactions. Another patient developed severe muscle cramps after taking 1,600 mg of Gleevec daily for 6 days. Complete resolution of muscle cramps occurred following interruption of therapy and treatment was subsequently resumed. Another patient that was prescribed 400 mg daily, took 800 mg of Gleevec on Day 1 and 1,200 mg on Day 2. Therapy was interrupted, no adverse reactions occurred and the patient resumed therapy.
Pediatric Overdose
One 3 year old male exposed to a single dose of 400 mg experienced vomiting, diarrhea, and anorexia; and another 3 year old male exposed to a single dose of 980 mg experienced decreased white blood cell (WBC) count and diarrhea.
5DESCRIPTION
Imatinib is a small molecule kinase inhibitor. Gleevec film-coated tablets are supplied as 100 mg and 400 mg tablets for oral administration. Each 100 mg tablet contains 119.5 mg of imatinib mesylate equivalent to 100 mg of imatinib free base. Each 400 mg tablet contains 478 mg of imatinib mesylate equivalent to 400 mg of imatinib free base. Imatinib mesylate is designated chemically as 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide methanesulfonate and its structural formula is:

Imatinib mesylate is a white to off-white to brownish or yellowish tinged crystalline powder. Its molecular formula is C
Inactive Ingredients: colloidal silicon dioxide (NF); crospovidone (NF); hydroxypropyl methylcellulose (USP); magnesium stearate (NF); and microcrystalline cellulose (NF). Tablet coating: ferric oxide, red (NF); ferric oxide, yellow (NF); hydroxypropyl methylcellulose (USP); polyethylene glycol (NF), and talc (USP).
6REFERENCES
OSHA Hazardous Drugs.
7HOW SUPPLIED/STORAGE AND HANDLING
Gleevec film-coated tablets are supplied as 100 mg and 400 mg tablets for oral administration. Each 100 mg tablet contains 119.5 mg of imatinib mesylate equivalent to 100 mg of imatinib free base. Each 400 mg tablet contains 478 mg of imatinib mesylate equivalent to 400 mg of imatinib free base.
- 100-mg tablets
Very dark yellow to brownish orange, film-coated tablets, round, biconvex with bevelled edges, debossed with “NVR” on one side, and “SA” with score on the other side.
Bottles of 90 tablets…………………………………NDC 0078-0401-34
- 400-mg tablets
Very dark yellow to brownish orange, film-coated tablets, ovaloid, biconvex with bevelled edges, debossed with “gleevec” on one side and score on the other side.
Unit Dose (blister pack of 30) ………………………NDC 0078-0649-30
Unit Dose (carton box including 3 blister packs of 10)…………NDC 0078-0649-13
Very dark yellow to brownish orange, film-coated tablets, ovaloid, biconvex with bevelled edges, debossed with “400” on one side and score on the other side with “SL” on each side of the score.
Unit Dose (carton box including 3 blister packs of 10)………....NDC 0078-1490-13
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight container, USP.
Do not crush Gleevec tablets. Avoid direct contact of crushed tablets with the skin or mucous membranes. If such contact occurs, wash thoroughly as outlined in the references. Avoid exposure to crushed tablets.
8PATIENT COUNSELING INFORMATION
Dosing and Administration
Advise patients to take Gleevec exactly as prescribed, not to change their dose or to stop taking Gleevec unless they are told to do so by their doctor. If the patient missed a dose of Gleevec, the patient should take the next scheduled dose at its regular time. The patient should not take two doses at the same time. Advise patients to take Gleevec with a meal and a large glass of water
Fluid Retention and Edema
Inform patients of the possibility of developing edema and fluid retention. Advise patients to contact their health care provider if unexpected rapid weight gain occurs
Hepatotoxicity
Inform patients of the possibility of developing liver function abnormalities and serious hepatic toxicity. Advise patients to immediately contact their health care provider if signs of liver failure occur, including jaundice, anorexia, bleeding, or bruising
Pregnancy and Breastfeeding
Advise patients to inform their doctor if they are or think they may be pregnant. Advise women of reproductive potential to avoid becoming pregnant while taking Gleevec. Female patients of reproductive potential taking Gleevec should use highly effective contraception during treatment and for fourteen days after stopping treatment with Gleevec
Drug Interactions
Gleevec and certain other medicines, such as warfarin, erythromycin, and phenytoin, including over-the-counter medications, such as herbal products, can interact with each other. Advise patients to tell their doctor if they are taking or plan to take iron supplements. Avoid grapefruit juice and other foods known to inhibit CYP3A4 while taking Gleevec
Pediatric
Advise patients that growth retardation has been reported in children and pre-adolescents receiving Gleevec. The long term effects of prolonged treatment with Gleevec on growth in children are unknown. Therefore, closely monitor growth in children under Gleevec treatment
Driving and Using Machines
Advise patients that they may experience side effects, such as dizziness, blurred vision, or somnolence during treatment with Gleevec. Therefore, caution patients about driving a car or operating machinery
Distributed by
© Novartis
T2025-70
9PRINCIPAL DISPLAY PANEL
NDC 0078-0401-34
Rx only
Gleevec
Tablets
100 mg
Each tablet contains 119.5 mg
90 Tablets
NOVARTIS

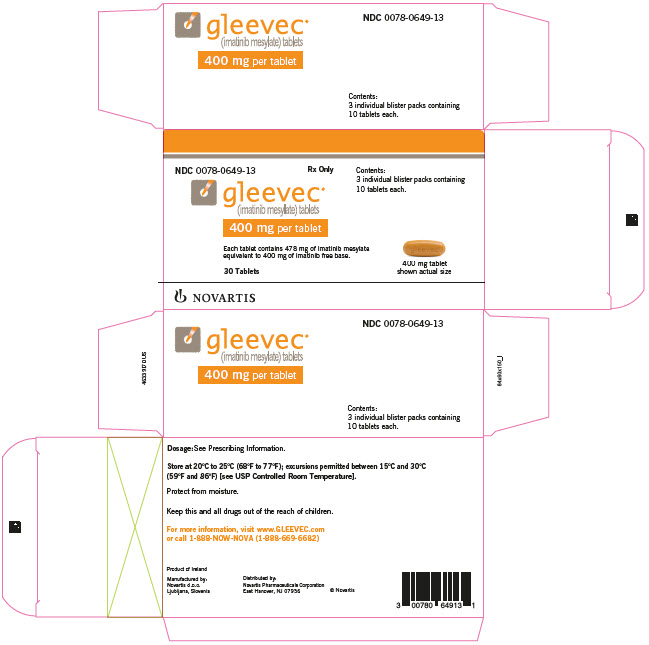
10PRINCIPAL DISPLAY PANEL
NDC 0078-0649-13
Rx Only
gleevec
400 mg per tablet
Each tablet contains 478 mg of imatinib mesylate
30 Tablets
NOVARTIS
11PRINCIPAL DISPLAY PANEL
NDC 0078-1490-13
Rx Only
gleevec
400 mg per tablet
Each tablet contains 478 mg of imatinib mesylate
30 Tablets
NOVARTIS
