Arixtra
What is Arixtra (Fondaparinux)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The main goal of this study is to compare two treatments in patients with a specific type of heart attack called Non-ST-elevation Myocardial Infarction (NSTEMI). The investigators want to find out whether using aspirin alone is as effective and safer than using aspirin together with a second blood thinner called fondaparinux. Both treatments will be given before a scheduled heart procedure called ...
Summary: Current management of intermediate- and high-risk pulmonary embolism is primarily based on curative subcutaneous or intravenous anticoagulation, with or without systemic fibrinolytic therapy or thrombectomy (8). Initial treatment with low-molecular-weight heparin (LMWH) or fondaparinux is preferred over unfractionated heparin (UFH) due to their lower risk of serious bleeding and heparin-induced th...
Summary: The goal of this clinical trial is to find out the clinical and cost effectiveness of Thromboprophylaxis in participants who have been placed in a plaster cast or splint after injury. The main questions it aims to answer are: * whether giving tablets to people at high risks of clots after a leg injury is as good as injections (standard care) * whether giving any medication after a leg injury is be...
Related Latest Advances
Brand Information
- use of indwelling epidural catheters
- concomitant use of other drugs that affect hemostasis, such as non-steroidal anti- inflammatory drugs (NSAIDs), platelet inhibitors, or other anticoagulants
- a history of traumatic or repeated epidural or spinal puncture
- a history of spinal deformity or spinal surgery
- Optimal timing between the administration of ARIXTRA and neuraxial procedures is not known.
- Severe renal impairment (creatinine clearance [CrCl] less than 30 mL/min)
- Active major bleeding.
- Bacterial endocarditis.
- Thrombocytopenia associated with a positive
- Body weight less than 50 kg (venous thromboembolism [VTE] prophylaxis in adults only)
- History of serious hypersensitivity reaction (e.g., angioedema, anaphylactoid/anaphylactic reactions) to ARIXTRA.
- Spinal or epidural hematomas
- Hemorrhage
- Renal impairment and bleeding risk
- Body weight less than 50 kg and bleeding risk
- Thrombocytopenia

- Advise patients that ARIXTRA should be given by subcutaneous injection. Instruct patients in the proper technique for administration.
- Instruct patients that if they miss a dose of ARIXTRA, to inject the dose as soon as they remember. Advise patients not to inject two doses at the same time.
- The most important risk with ARIXTRA administration is bleeding. Counsel patients on signs and symptoms of possible bleeding.
- Advise patients that it may take them longer than usual to stop bleeding.
- Advise patients that they may bruise and/or bleed more easily when they are treated with ARIXTRA.
- Advise patients to report any unusual bleeding, bruising, or signs of thrombocytopenia (such as a rash of dark red spots under the skin) to their physician
- Advise patients to tell their physicians and dentists they are taking ARIXTRA and/or any other product known to affect bleeding before any surgery is scheduled and before any new drug is taken
- Advise patients to tell their physicians and dentists of all medications they are taking, including those obtained without a prescription, such as aspirin or other NSAIDs
- ARIXTRA is available in different types of syringes. Confirm the syringe looks like the figure below before you continue:
- Your healthcare provider should show you how to prepare and inject ARIXTRA.
- Each ARIXTRA syringe is 1 dose of ARIXTRA.
- Use ARIXTRA exactly as prescribed by your doctor.
- The packaging (needle guard) for ARIXTRA contains dry natural latex rubber.
- Store ARIXTRA at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep ARIXTRA syringes and all medicines out of the reach of children.
- the solution appears discolored (the solution should normally appear clear)
- you see any particles in the solution
- the syringe is damaged
- it is out of date (expired)
- ARIXTRA is available in different types of syringes. Confirm the syringe looks like the figure below before you continue:

- Your healthcare provider should show you how to prepare and inject ARIXTRA. Do not inject your child until you have been shown how to inject ARIXTRA.
- Each ARIXTRA syringe is 1 dose of ARIXTRA.
- Use ARIXTRA exactly as prescribed by your doctor.
- Store ARIXTRA syringes in a refrigerator between 36°F to 46°F (2°C to 8°C) for up to the beyond use date on the syringe.
- Do not freeze.
- Store syringes in a clean container in the refrigerator.
- Do not store ARIXTRA syringes at room temperature between 68°F to 77°F (20°C to 25°C).
- Take ARIXTRA syringe out of the refrigerator and allow it to reach room temperature. Inject right away after the syringe reaches room temperature.
- Throw away (dispose of) ARIXTRA syringes that has been left at room temperature for longer than 4 hours.
- Keep ARIXTRA syringes and all medicines out of the reach of children.
- the solution appears discolored (the solution should normally appear clear)
- you see any particles in the solution
- the syringe is damaged, including:
- past the beyond use date on the syringe labeled by the pharmacy

in 0.4 mL of an isotonic solution of sodium chloride and water for injection. May also
contain sodium hydroxide and/or hydrochloric acid as pH adjusters.

in 0.6 mL of an isotonic solution of sodium chloride and water for injection. May also
contain sodium hydroxide and/or hydrochloric acid as pH adjusters.
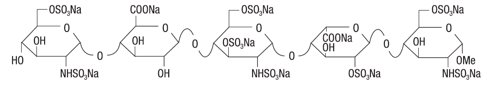
in 0.8 mL of an isotonic solution of sodium chloride and water for injection. May also
contain sodium hydroxide and/or hydrochloric acid as pH adjusters.



