Brand Name
Valstar
Generic Name
Valrubicin
View Brand Information FDA approval date: October 01, 1998
Form: Solution
What is Valstar (Valrubicin)?
Valrubicin intravesical solution is an anthracycline topoisomerase inhibitor indicated for intravesical therapy of BCG-refractory carcinoma in situ of the urinary bladder in patients for whom immediate cystectomy would be associated with unacceptable morbidity or mortality. Valrubicin intravesical solution is an anthracycline topoisomerase inhibitor indicated for intravesical therapy of BCG-refractory carcinoma in situ of the urinary bladder in patients for whom immediate cystectomy would be associated with unacceptable morbidity or mortality.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Valstar (valrubicin)
1INDICATIONS AND USAGE
VALSTAR is an anthracycline topoisomerase inhibitor indicated for intravesical therapy of BCG-refractory carcinoma
2DOSAGE FORMS AND STRENGTHS
200 mg/5 mL sterile, clear red, solution in single-use vials for intravesical instillation upon dilution.
3CONTRAINDICATIONS
VALSTAR is contraindicated in patients with:
- Perforated bladder [
- Known hypersensitivity to anthracyclines or polyoxyl castor oil
- Active urinary tract infection
- Small bladder capacity and unable to tolerate a 75 mL instillation
4DRUG INTERACTIONS
No drug interaction studies were conducted.
5OVERDOSAGE
There is no known antidote for overdoses of VALSTAR. The primary anticipated complications of overdosage associated with intravesical administration would be consistent with irritable bladder symptoms.
Myelosuppression is possible if VALSTAR is inadvertently administered systemically or if significant systemic exposure occurs following intravesical administration (e.g., in patients with bladder rupture/perforation). Under such inadvertent exposures in the peritoneal cavity, the expected toxicities include leukopenia and neutropenia, beginning within 1 week of dose administration, with nadirs by the second week, and recovery generally by the third week. If VALSTAR is administered when bladder rupture or perforation is suspected, weekly monitoring of complete blood counts should be performed for 3 weeks.
6DESCRIPTION
VALSTAR contains valrubicin (N-trifluoroacetyladriamycin-14-valerate), which is a semisynthetic analog of the anthracycline doxorubicin as a cytotoxic agent. The chemical name of valrubicin is (2
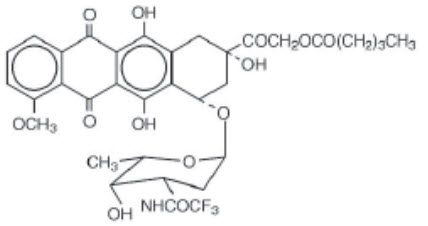
VALSTAR is intended for intravesical administration in the urinary bladder. It is supplied as a nonaqueous solution that should be diluted before intravesical administration. Each vial of VALSTAR contains 200 mg valrubicin at a concentration of 40 mg/mL in 5 mL of 50% polyoxyl castor oil/50% dehydrated alcohol, USP without preservatives or other additives. The solution is sterile and nonpyrogenic.
7CLINICAL STUDIES
VALSTAR was administered intravesically to a total of 230 patients with transitional cell carcinoma of the bladder, including 205 patients who received multiple weekly doses ranging from 200 to 900 mg. One hundred seventy-nine of the 205 patients received the approved dose and schedule of 800 mg weekly for multiple weeks. Patients receiving VALSTAR for refractory carcinoma in situ were monitored for disease recurrence or progression with cystoscopy, biopsy, and urine cytology every 3 months.
In the 90 study patients with BCG-refractory carcinoma
After intravesical administration of VALSTAR, 16 patients (18%) had a complete response documented by bladder biopsies and cytology at 6 months following initiation of therapy. Median duration of response from start of treatment varied according to the method of analysis (13.5 months if measured to last bladder biopsy without tumor and 21 months if measured until time of documented recurrence). A retrospective analysis in the 16 patients with complete response to VALSTAR demonstrated that time to recurrence of their disease after treatment with VALSTAR was longer than time to recurrence after previous courses of intravesical therapy.
Of the 90 patients with BCG-refractory CIS, 11% (10 patients) developed metastatic or deeply-invasive bladder cancer during follow-up; four of these patients, none who underwent cystectomy, died with metastatic bladder cancer and six were found to have developed stage progression to deeply-invasive disease (T3), with lymph node involvement in one patient, at the time of cystectomy. It is uncertain to what extent the development of advanced bladder cancer in these patients was due to the delay in cystectomy required to receive treatment with VALSTAR (3 months was the time of follow-up to determine response), as cystectomy was often delayed or was never performed despite failure of treatment with VALSTAR. In the 10 patients documented to have invasive bladder cancer or metastatic disease, the delay between the time of treatment failure (when cystectomy should have been performed) and cystectomy or documentation of advanced bladder cancer was a median of 17.5 months.
8REFERENCES
- OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
9HOW SUPPLIED/STORAGE AND HANDLING
VALSTAR is a sterile, clear red solution in polyoxyl castor oil/dehydrated alcohol, USP, containing 40 mg valrubicin per mL. VALSTAR is available in single-use, clear glass vials, individually packaged in the following sizes:
NDC 67979-001-01 Carton of four 200 mg/5 mL single-use vials
Store vials under refrigeration at 2°-8°C (36°-46°F) in the carton. DO NOT FREEZE.
10PATIENT COUNSELING INFORMATION
Risk of Metastatic Bladder Cancer with Delayed Cystectomy
- Inform patients that VALSTAR has been shown to induce complete responses in only about 1 in 5 patients, and that delaying cystectomy could lead to development of metastatic bladder cancer, which is lethal. Discuss the relative risk of cystectomy versus the risk of metastatic bladder cancer
Local Adverse Reactions Before and During Treatment
- Inform patients that the major acute toxicities from VALSTAR are related to irritable bladder symptoms that may occur during instillation and retention of VALSTAR and for a limited period following voiding
- Inform patients that for the first 24 hours following administration, red-tinged urine is typical.
- Advise patients to report prolonged irritable bladder symptoms or prolonged passage of red-colored urine immediately to their physician.
- Instruct patients to maintain adequate hydration following VALSTAR treatment.
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception during treatment with VALSTAR and for 6 months after the last dose. Advise females to inform their healthcare provider of a known or suspected pregnancy
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment with VALSTAR and for 3 months after the last dose
Lactation
- Advise females not to breastfeed during treatment with VALSTAR and for 2 weeks after the last dose
Healthcare professionals can telephone Endo (1-800-462-3636) for information on this product.
Manufactured for:
Manufactured by:
© 2024 Endo, Inc. or one of its affiliates.
Revised: 07/2024
11Package Label – Principle Display Panel – Carton – 4 Vials
