Generic Name
RisperiDONE
Brand Names
UZEDY, Risperdal M-TAB, Perseris, Risperdal Consta, Risperdal, Rykindo
FDA approval date: December 29, 1993
Classification: Atypical Antipsychotic
Form: Injection, Tablet, Kit, Solution
What is UZEDY (RisperiDONE)?
Risperidone orally disintegrating tablets are atypical antipsychotic indicated for: Treatment of schizophrenia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
UZEDY (risperidone)
WARNING: INCREASED MORTALITY INELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. UZEDY is not approved for the treatment of patients with dementia-related psychosis and has not been studied in this patient population
1DOSAGE FORMS AND STRENGTHS
Extended-release injectable suspension: sterile, white to off-white opaque viscous suspension available in the following strengths of risperidone: 50 mg/0.14 mL, 75 mg/0.21 mL, 100 mg/0.28 mL, 125 mg/0.35 mL, 150 mg/0.42 mL, 200 mg/0.56 mL, and 250 mg/0.7 mL.
2CONTRAINDICATIONS
UZEDY is contraindicated in patients with a known hypersensitivity to risperidone, its metabolite, paliperidone, or to any of its components. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with risperidone or paliperidone.
3ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Increased mortality in elderly patients with dementia-related psychosis
- Cerebrovascular adverse events, including stroke, in elderly patients with dementia-related psychosis
- Neuroleptic malignant syndrome
- Tardive dyskinesia
- Metabolic changes
- Hyperprolactinemia
- Orthostatic hypotension and syncope
- Falls
- Leukopenia, neutropenia and agranulocytosis
- Potential for cognitive and motor impairment
- Seizures
- Dysphagia
- Priapism
- Body temperature regulation
3.1Clinical TrialsExperience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Treatment of Schizophrenia in Adults
UZEDY is to be administered subcutaneously
The data described in this section are derived from a clinical trial database consisting of 9,803 patients exposed to one or more doses of oral risperidone for the treatment of schizophrenia and other psychiatric disorders. Of these 9,803 patients, 2,687 were patients who received oral risperidone while participating in double-blind, placebo-controlled trials. The conditions and duration of treatment with oral risperidone varied greatly and included (in overlapping categories) double-blind, fixed- and flexible-dose, placebo- or active-controlled studies and open-label phases of studies, inpatients and outpatients, and short-term (up to 12 weeks) and longer-term (up to 3 years) exposures. Safety was assessed by collecting adverse reactions and performing physical examinations, vital signs, body weights, laboratory analyses, and ECGs.
Injection site reactions for UZEDY presented in this section (see “Injection Site Reactions with UZEDY” below) are based on a randomized withdrawal study in patients with schizophrenia consisting of a 12-week open-label oral risperidone (2 mg to 5 mg) stabilization phase, followed by a placebo-controlled phase in which patients were randomized to UZEDY (once monthly or once every 2 months) or placebo for a variable time until impending relapse or study completion
The safety of UZEDY was evaluated in a total of 740 adult patients with schizophrenia who received at least 1 dose of UZEDY during the clinical development program. A total of 351 patients were exposed to UZEDY for at least 6 months, of which 221 patients were exposed to UZEDY for at least 12 months, which included 112 patients exposed to once monthly and 109 patients to once every 2 months dosing regimens. In addition, 32 patients were exposed to UZEDY for at least 24 months.
Treatment of Bipolar Disorder in Adults
UZEDY is to be administered subcutaneously
The safety of UZEDY as monotherapy or as adjunctive therapy to lithium or valproate for the maintenance treatment of Bipolar I Disorder in adults is based on adequate and well-controlled studies of another risperidone long-acting injection given once every 2 weeks. The results of those adequate and well-controlled studies are presented below.
Safety data are presented from a trial assessing the efficacy and safety of another risperidone long-acting injection, given once every 2 weeks, when administered as monotherapy for maintenance treatment in patients with bipolar I disorder.
Safety data are also presented from a trial assessing the efficacy and safety of another risperidone long-acting injection, given once every 2 weeks, administered as adjunctive maintenance treatment in patients with bipolar disorder.
Adverse Reactions in Studies with Oral Risperidone
The most common adverse reactions in clinical trials of oral risperidone (>5% and twice placebo) were parkinsonism, akathisia, dystonia, tremor, sedation, dizziness, anxiety, blurred vision, nausea, vomiting, upper abdominal pain, stomach discomfort, dyspepsia, diarrhea, salivary hypersecretion, constipation, dry mouth, increased appetite, increased weight, fatigue, rash, nasal congestion, upper respiratory tract infection, nasopharyngitis, and pharyngolaryngeal pain.
Commonly-Observed Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials – Adult Patients with Schizophrenia Treated with Oral Risperidone
Table 7 lists the adverse reactions reported in 2% or more of oral risperidone-treated adult patients with schizophrenia in three 4- to 8-week, double-blind, placebo-controlled trials.
* Parkinsonism includes extrapyramidal disorder, musculoskeletal stiffness, parkinsonism, cogwheel rigidity, akinesia, bradykinesia, hypokinesia, masked facies, muscle rigidity, and Parkinson’s disease. Akathisia includes akathisia and restlessness. Dystonia includes dystonia, muscle spasms, muscle contractions involuntary, muscle contracture, oculogyration, tongue paralysis. Tremor includes tremor and parkinsonian rest tremor.
Adverse Reactions in Studies with Another Long-Acting Injection Risperidone, Given Once Every 2 Weeks
The most common adverse reactions in the double-blind, placebo-controlled periods of the bipolar disorder trials of another risperidone long-acting injection were weight increased (5% in the monotherapy trial) and tremor and parkinsonism (≥ 10% in the adjunctive treatment trial).
Commonly-Observed Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials – Adult Patients with Bipolar Disorder Treated with Another Risperidone Long-Acting Injection Given Once Every 2 Weeks
Table 8 lists the treatment-emergent adverse reactions reported in 2% or more of patients treated with another risperidone long-acting injection, given once every 2 weeks, in the 24-month double-blind, placebo-controlled treatment period of the trial assessing the efficacy and safety of another risperidone long-acting injection when administered as monotherapy for maintenance treatment in patients with bipolar I disorder.
a The data presented are from a study with another risperidone long-acting injection that was administered once every 2 weeks, intramuscularly. UZEDY is to be administered subcutaneously.
Table 9 lists the treatment-emergent adverse reactions reported in 4% or more of patients in the 52-week double-blind, placebo-controlled treatment phase of a trial assessing the efficacy and safety of another Risperidone Long-Acting Injection, given once every 2 weeks, when administered as adjunctive maintenance treatment in patients with bipolar disorder.
a The data presented are from a study with another risperidone long-acting injection that was administered once every 2 weeks, intramuscularly. UZEDY is to be administered subcutaneously.
b Patients received double-blind treatment with another risperidone long-acting injection, given once every 2 weeks, or placebo in addition to continuing their treatment as usual, which included mood stabilizers, antidepressants, and/or anxiolytics.
c Parkinsonism includes muscle rigidity, hypokinesia, cogwheel rigidity, and bradykinesia.
d Dyskinesia includes muscle twitching and dyskinesia.
e Sedation includes sedation and somnolence.
b Patients received double-blind treatment with another risperidone long-acting injection, given once every 2 weeks, or placebo in addition to continuing their treatment as usual, which included mood stabilizers, antidepressants, and/or anxiolytics.
c Parkinsonism includes muscle rigidity, hypokinesia, cogwheel rigidity, and bradykinesia.
d Dyskinesia includes muscle twitching and dyskinesia.
e Sedation includes sedation and somnolence.
Other Adverse Reactions Observed During the Clinical Trial Evaluations of Oral Risperidone
The following is a list of additional adverse drug reactions that have been reported during the clinical trial evaluation of oral risperidone:
Blood and Lymphatic System Disorders: anemia, granulocytopenia, neutropenia
Cardiac Disorders: sinus bradycardia, sinus tachycardia, atrioventricular block first degree, bundle branch block left, bundle branch block right, atrioventricular block
Ear and Labyrinth Disorders: ear pain, tinnitus
Endocrine Disorders:hyperprolactinemia
Eye Disorders: ocular hyperemia, eye discharge, conjunctivitis, eye rolling, eyelid edema, eye swelling, eyelid margin crusting, dry eye, lacrimation increased, photophobia, glaucoma, visual acuity reduced
Gastrointestinal Disorders: dysphagia, fecaloma, fecal incontinence, gastritis, lip swelling, cheilitis, aptyalism
General Disorders: edema peripheral, thirst, gait disturbance, chest discomfort, chest pain, influenza-like illness, pitting edema, edema, chills, sluggishness, malaise, face edema, discomfort, generalized edema, drug withdrawal syndrome, peripheral coldness, feeling abnormal
Immune System Disorders: drug hypersensitivity
Infections and Infestations: pneumonia, influenza, ear infection, viral infection, pharyngitis, tonsillitis, bronchitis, eye infection, localized infection, cystitis, cellulitis, otitis media, onychomycosis, acarodermatitis, bronchopneumonia, respiratory tract infection, tracheobronchitis, otitis media chronic
Investigations: body temperature increased, blood prolactin increased, alanine aminotransferase increased, electrocardiogram abnormal, eosinophil count increased, white blood cell count decreased, blood glucose increased, hemoglobin decreased, hematocrit decreased, body temperature decreased, blood pressure decreased, transaminases increased
Metabolism and Nutrition Disorders: decreased appetite, polydipsia, anorexia
Musculoskeletal, Connective Tissue, and Bone Disorders: joint swelling, joint stiffness, musculoskeletal chest pain, posture abnormal, myalgia, neck pain, muscular weakness, muscle rigidity, rhabdomyolysis, torticollis
Nervous System Disorders: balance disorder, disturbance in attention, dysarthria, unresponsive to stimuli, depressed level of consciousness, movement disorder, transient ischemic attack, coordination abnormal, cerebrovascular accident, speech disorder, syncope, loss of consciousness, hypoesthesia, tardive dyskinesia, cerebral ischemia, cerebrovascular disorder, neuroleptic malignant syndrome, diabetic coma, head titubation
Psychiatric Disorders: agitation, blunted affect, confusional state, middle insomnia, nervousness, sleep disorder, listlessness, libido decreased, anorgasmia
Renal and Urinary Disorders: enuresis, dysuria, pollakiuria, urinary incontinence
Reproductive System and Breast Disorders: menstruation irregular, amenorrhea, gynecomastia, galactorrhea, vaginal discharge, menstrual disorder, erectile dysfunction, retrograde ejaculation, ejaculation disorder, sexual dysfunction, breast enlargement
Respiratory, Thoracic, and Mediastinal Disorders: wheezing, pneumonia aspiration, sinus congestion, dysphonia, productive cough, pulmonary congestion, respiratory tract congestion, rales, respiratory disorder, hyperventilation, nasal edema
Skin and Subcutaneous Tissue Disorders: erythema, skin discoloration, skin lesion, pruritus, skin disorder, rash erythematous, rash papular, acne, hyperkeratosis, seborrheic dermatitis, rash generalized, rash maculopapular
Vascular Disorders: hypotension, flushing
Additional Adverse Reactions Reported With Another Risperidone Long-Acting Injection, Given Once Every 2 Weeks
The following is a list of additional adverse reactions that have been reported during the premarketing evaluation of another risperidone long-acting injection, given once every 2 weeks, regardless of frequency of occurrence:
Cardiac Disorders: bradycardia, tachycardia, palpitations
Ear and Labyrinth Disorders: vertigo
Eye Disorders: blepharospasm
Gastrointestinal Disorders: toothache, tongue spasm, diarrhea, vomiting, abdominal pain upper, abdominal pain, stomach discomfort
General Disorders and Administration Site Conditions: pain, injection site pain, induration, injection site induration, injection site swelling, injection site reaction
Infections and Infestations: lower respiratory tract infection, infection, gastroenteritis, subcutaneous abscess, nasopharyngitis, urinary tract infection, rhinitis, sinusitis
Injury and Poisoning: fall, procedural pain
Investigations: weight decreased, gamma-glutamyltransferase increased, hepatic enzyme increased, aspartate aminotransferase increased, electrocardiogram QT prolonged, glucose urine present
Musculoskeletal, Connective Tissue, and Bone Disorders: buttock pain, back pain
Nervous System Disorders: convulsion, paresthesia, dystonia, drooling, dizziness postural, akinesia, hypokinesia
Psychiatric Disorders: depression, insomnia, anxiety, initial insomnia
Reproductive System and Breast Disorders: oligomenorrhea, breast discomfort, menstruation delayed, ejaculation delayed
Respiratory, Thoracic, and Mediastinal Disorders: nasal congestion, pharyngolaryngeal pain, dyspnea, rhinorrhea
Skin and Subcutaneous Tissue Disorders: eczema, rash, pruritis generalized
Vascular Disorders: hypertension, orthostatic hypotension
Discontinuations Due to Adverse Drug Reactions with Oral Risperidone
Schizophrenia
Approximately 7% (39/564) of oral risperidone-treated patients in double-blind, placebo-controlled trials discontinued treatment due to an adverse reaction, compared with 4% (10/225) who were receiving placebo. The adverse reactions associated with discontinuation in 2 or more oral risperidone-treated patients were:
Discontinuation for extrapyramidal symptoms (including Parkinsonism, akathisia, dystonia, and tardive dyskinesia) was 1% in placebo-treated patients, and 3.4% in active control-treated patients in a double-blind, placebo- and active-controlled trial.
Discontinuations Due to Adverse Drug Reactions with Another Long-Acting Injection Risperidone, Given Once Every 2 Weeks
Bipolar Disorder
In a 24-month double-blind, placebo-controlled treatment period of the trial assessing the efficacy and safety of another risperidone long-acting injection, given once every 2 weeks, monotherapy for maintenance treatment in patients with bipolar I disorder, 1 (0.6%) of 154 patients treated with another risperidone long-acting injection, given once every 2 weeks, discontinued due to an adverse reaction (hyperglycemia).
In the 52-week double-blind phase of the placebo-controlled trial in which patients treated with another risperidone long-acting injection, given once every 2 weeks, was administered as adjunctive therapy to patients with bipolar disorder in addition to continuing with their usual treatment, approximately 4% (3/72) of patients treated with another risperidone long-acting injection, given once every 2 weeks, discontinued treatment due to an adverse event, compared with 1.5% (1/67) of placebo-treated patients. Adverse reactions associated with discontinuation in patients treated with another risperidone long-acting injection, given once every 2 weeks, were hypokinesia (one patient) and tardive dyskinesia (one patient).
Dose Dependency of Adverse Reactions in Clinical Trials of Oral Risperidone
Extrapyramidal Symptoms
Data from two fixed-dose trials in adults with schizophrenia provided evidence of dose-relatedness for extrapyramidal symptoms associated with oral risperidone treatment. Two methods were used to measure extrapyramidal symptoms (EPS) in an 8-week trial comparing 4 fixed doses of oral risperidone (2, 6, 10, and 16 mg/day), including (1) a Parkinsonism score (mean change from baseline) from the Extrapyramidal Symptom Rating Scale, and (2) incidence of spontaneous complaints of EPS:
Similar methods were used to measure extrapyramidal symptoms (EPS) in an 8-week trial comparing 5 fixed doses of oral risperidone (1, 4, 8, 12, and 16 mg/day):
Changes in Body Weight
Weight gain was observed in short-term, controlled trials and longer-term uncontrolled studies in adults
Dystonia
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Other Adverse Reactions
Adverse reaction data elicited by a checklist for side effects from a large study comparing 5 fixed doses of oral risperidone (1, 4, 8, 12, and 16 mg/day) were explored for dose-relatedness of adverse events. A Cochran-Armitage Test for trend in these data revealed a positive trend (p<0.05) for the following adverse reactions: somnolence, vision abnormal, dizziness, palpitations, weight increase, erectile dysfunction, ejaculation disorder, sexual function abnormal, fatigue, and skin discoloration.
Changes in ECG
Between-group comparisons for pooled placebo-controlled trials of oral risperidone in adults revealed no statistically significant differences between oral risperidone and placebo in mean changes from baseline in ECG parameters, including QT, QTc, and PR intervals, and heart rate. When all oral risperidone doses were pooled from randomized controlled trials in several indications, there was a mean increase in heart rate of 1 beat per minute compared to no change for placebo patients. In short-term schizophrenia trials, higher doses of oral risperidone were associated with a higher mean increase in heart rate compared to placebo (4 to 6 beats per minute).
The ECGs of 227 patients with Bipolar I Disorder were evaluated in the 24-month double-blind, placebo-controlled period. There were no clinically relevant differences in QTc intervals (using Fridericia's and linear correction factors) during treatment with another risperidone long-acting injection, given once every 2 weeks, compared to placebo.
The ECGs of 85 patients with bipolar disorder were evaluated in the 52-week double-blind, placebo-controlled trial. There were no statistically significant differences in QTc intervals (using Fridericia's and linear correction factors) during adjunctive treatment with either another risperidone long-acting injection (25 mg, 37.5 mg, or 50 mg), given once every 2 weeks, or placebo in addition to treatment as usual.
Injection Site Reactions with UZEDY
Local tolerability assessments were administered to patients who reported injection site adverse reactions in a randomized withdrawal study with UZEDY in adult patients with schizophrenia. The injection site was assessed by appropriately trained personnel throughout the clinical development program.
All injection site reactions (nodule, pruritus, erythema, mass, and swelling) were mild to moderate in severity with the exception of 1 case of severe pruritus which resolved after 6 days. Injection site reactions were reported in 22 patients (13%) in the placebo group, 36 patients (20%) in the UZEDY once monthly group, and 37 patients (21%) in the UZEDY once every 2 months group. The most common injection site reactions were: nodule (7% in each UZEDY-treated group and 3% in the placebo group) and pruritus (5% and 3% in the UZEDY-treated once monthly and once every 2 months groups, respectively, and 2% in the placebo group).
3.2PostmarketingExperience
The following adverse reactions have been identified during post-approval use of oral risperidone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These adverse reactions include: alopecia, anaphylactic reaction, angioedema, atrial fibrillation, cardiopulmonary arrest, catatonia, diabetic ketoacidosis in patients with impaired glucose metabolism, dysgeusia, hypoglycemia, hypothermia, ileus, inappropriate antidiuretic hormone secretion, intestinal obstruction, jaundice, mania, pancreatitis, pituitary adenoma, precocious puberty, pulmonary embolism, QT prolongation, sleep apnea syndrome, somnambulism, Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), sudden death, thrombocytopenia, thrombotic thrombocytopenic purpura, urinary retention, and water intoxication.
In addition, the following adverse reactions have been observed during postapproval use of UZEDY: injection site pain.
In addition, the following adverse reactions have been observed during postapproval use of another risperidone long-acting injection, given intramuscularly once every 2 weeks: cerebrovascular disorders, including cerebrovascular accidents, and diabetes mellitus aggravated.
Retinal artery occlusion after injection of another risperidone long-acting injection, given intramuscularly once every 2 weeks, has been reported during postmarketing surveillance. This has been reported in the presence of abnormal arteriovenous anastomosis.
Serious injection site reactions including abscess, cellulitis, cyst, hematoma, necrosis, nodule, and ulcer have been reported with another risperidone long-acting injection, given intramuscularly once every 2 weeks, during postmarketing surveillance. Isolated cases required surgical intervention.
Very rarely, cases of anaphylactic reaction after injection with another risperidone long-acting injection, given intramuscularly once every 2 weeks, have been reported during postmarketing experience in patients who have previously tolerated oral risperidone.
Postmarketing cases of extrapyramidal symptoms (dystonia and dyskinesia) have been reported in patients concomitantly taking methylphenidate and risperidone when there was an increase or decrease in dosage, initiation, or discontinuation of either or both medications.
4DRUG INTERACTIONS
The interactions of UZEDY with co-administration of other drugs have not been studied. The drug interaction data provided in this section is based on studies with oral risperidone.
4.1Drugs HavingClinically Important Interactions with UZEDY
Table 13 includes clinically significant drug interactions with UZEDY.
4.2Drugs Having NoClinically Important Interactions with UZEDY
Based on pharmacokinetic studies with oral risperidone, no dosage adjustment of UZEDY is required when administered concomitantly with amitriptyline, cimetidine, ranitidine, clozapine, topiramate, and moderate CYP3A4 inhibitors (erythromycin). Additionally, no dosage adjustment is necessary for lithium, valproate, topiramate, digoxin, and CYP2D6 substrates (donepezil and galantamine) when co-administered with UZEDY
5OVERDOSAGE
Human Experience
No cases of overdose were reported in premarketing studies with UZEDY.
In premarketing experience with oral risperidone, there were eight reports of acute risperidone overdosage with estimated doses ranging from 20 to 300 mg and no fatalities. In general, reported signs and symptoms were those resulting from an exaggeration of the drug's known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, and extrapyramidal symptoms. One case, involving an estimated overdose of 240 mg, was associated with hyponatremia, hypokalemia, prolonged QT, and widened QRS. Another case, involving an estimated overdose of 36 mg, was associated with a seizure.
Postmarketing experience with oral risperidone included reports of acute overdosage with estimated doses of up to 360 mg. In general, the most frequently reported signs and symptoms are those resulting from an exaggeration of the drug's known pharmacological effects, i.e., drowsiness, sedation, tachycardia, hypotension, and extrapyramidal symptoms. Other postmarketing adverse reactions related to oral risperidone overdose include prolonged QT interval and convulsions. Torsade de pointes has been reported in association with combined overdose of oral risperidone and paroxetine.
Management of Overdosage
There is no specific antidote to risperidone. Provide supportive care including close medical supervision and monitoring. Treatment should consist of general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdosage. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs.
Consider contacting the Poison Help Line (1-800-222-1222) or medical toxicologist for additional overdosage management recommendations.
6DESCRIPTION
UZEDY contains risperidone, an atypical antipsychotic. Risperidone belongs to the chemical class of benzisoxazole derivatives. The chemical designation is 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl) piperidin-1yl] ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a] pyrimidin-4-one. Its molecular formula is C
The structural formula is:
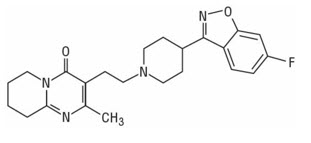
Risperidone is a white to off-white powder. It is practically insoluble in water and soluble in methanol and 0.1 N HCl. It has the following pKa values: 8.28 (piperidine moiety) and 3.12 (pyrimidine moiety).
UZEDY (risperidone) extended-release injectable suspension, for subcutaneous use, is a sterile, preservative free, white to off-white opaque viscous suspension. It is available in the following strengths (and deliverable volumes from a single-dose prefilled syringe): 50 mg (0.14 mL), 75 mg (0.21 mL), 100 mg (0.28 mL), 125 mg (0.35 mL), 150 mg (0.42 mL), 200 mg (0.56 mL), and 250 mg (0.7 mL). The inactive ingredients include dimethyl sulfoxide (45% w/w), methoxy-poly(ethylene glycol)-
7HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
UZEDY (risperidone) extended-release injectable suspension, for subcutaneous use, is a sterile, preservative free, white to off-white opaque viscous suspension.
UZEDY is supplied in single-dose kits as follows:
- 50 mg/0.14 mL single-dose prefilled syringe, packaged in a carton with one 21 gauge, 5/8-inch needle (NDC 51759-305-10)
- 75 mg/0.21 mL single-dose prefilled syringe, packaged in a carton with one 21 gauge, 5/8-inch needle (NDC 51759-410-10)
- 100 mg/0.28 mL single-dose prefilled syringe, packaged in a carton with one 21 gauge, 5/8-inch needle (NDC 51759-520-10)
- 125 mg/0.35 mL single-dose prefilled syringe, packaged in a carton with one 21 gauge, 5/8-inch needle (NDC 51759-630-10)
- 150 mg/0.42 mL single-dose prefilled syringe, packaged in a carton with one 21 gauge, 5/8-inch needle (NDC 51759-740-10)
- 200 mg/0.56 mL single-dose prefilled syringe, packaged in a carton with one 21 gauge, 5/8-inch needle (NDC 51759-850-10)
- 250 mg/0.7 mL single-dose prefilled syringe, packaged in a carton with one 21 gauge, 5/8-inch needle (NDC 51759-960-10)
The prefilled syringe cap is not made with natural rubber latex.
Storage and Handling
Store in refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
UZEDY may be stored in unopened original packaging at room temperature, 20°C to 25°C (68°F to 77°F), for up to 90 days. If unopened, UZEDY may be returned to refrigerated storage within 90 days. Once the carton is opened, administer UZEDY or discard.
8PATIENT COUNSELING INFORMATION
Neuroleptic Malignant Syndrome (NMS)
Counsel patients about a potentially fatal adverse reaction, Neuroleptic Malignant Syndrome (NMS) that has been reported in association with administration of antipsychotic drugs. Advise patients, family members, or caregivers to contact the healthcare provider or report to the emergency room if they experience signs and symptoms of NMS
Tardive Dyskinesia
Counsel patients on the signs and symptoms of tardive dyskinesia and to contact their healthcare provider if these abnormal movements occur
Metabolic Changes
Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia and diabetes mellitus and the need for specific monitoring, including blood glucose, lipids, and weight
Hyperprolactinemia
Counsel patients on signs and symptoms of hyperprolactinemia that may be associated with chronic use of UZEDY. Advise them to seek medical attention if they experience any of the following: amenorrhea or galactorrhea in females, erectile dysfunction, or gynecomastia in males
Orthostatic Hypotension and Syncope
Educate patients about the risk of orthostatic hypotension and syncope, particularly at the time of initiating treatment, re-initiating treatment or increasing the dose
Leukopenia/Neutropenia
Advise patients with a pre-existing low WBC or a history of drug-induced leukopenia/neutropenia that they should have their CBC monitored while being treated with UZEDY
Potential for Cognitive and Motor Impairment
Inform patients that UZEDY has the potential to impair judgement, thinking, and motor skills. Caution patients about performing activities requiring mental alertness, such as operating hazardous machinery, or operating a motor vehicle, until they are reasonably certain that UZEDY therapy does not affect them adversely
Priapism
Advise patients of the possibility of painful or prolonged penile erections (priapism). Instruct the patient to seek immediate medical attention in the event of priapism
Heat Exposure and Dehydration
Educate patients regarding appropriate care in avoiding overheating and dehydration
Concomitant Medication
Advise patients to inform their healthcare providers if they are taking, or plan to take, any prescription or over-the-counter drugs, as there is a potential for interaction
Alcohol
Advise patients to avoid alcohol during treatment with UZEDY
Pregnancy
Advise patients to notify their healthcare professional if they become pregnant or intend to become pregnant during treatment with UZEDY. Advise patients that UZEDY may cause extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder) in a neonate. Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to UZEDY during pregnancy
Lactation
Advise breastfeeding women using UZEDY to monitor infants for somnolence, failure to thrive, jitteriness, and extrapyramidal symptoms (tremors and abnormal muscle movements) and to seek medical care if they notice these signs
Infertility
Advise females of reproductive potential that UZEDY may impair fertility due to an increase in serum prolactin levels. The effects on fertility are reversible
UZE-005
Manufactured for:
©2025 Teva Neuroscience, Inc.
9Package/Label Display Panel
1 month dosing
For administration by a healthcare professional
Rx Only
UZEDY® (risperiDONE) extended-release injectable suspension
FOR SUBCUTANEOUS USE ONLY
NDC 51759-305-10
One single-dose prefilled syringe
50 mg/0.14 mL administered every one month
One single-dose prefilled syringe
50 mg/0.14 mL administered every one month
Store in refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Allow UZEDY to
UZEDY may be stored in unopened original packaging at room temperature, 20°C to 25°C (68°F to 77°F), for up to 90 days. If unopened, UZEDY may be returned to refrigerated storage within 90 days.
Once the carton is opened, administer UZEDY or discard.
OPEN HERE

10Package/Label Display Panel
1 month dosing
For administration by a healthcare professional
Rx Only
UZEDY® (risperiDONE) extended-release injectable suspension
FOR SUBCUTANEOUS USE ONLY
FOR SUBCUTANEOUS USE ONLY
NDC 51759-410-10
One single-dose prefilled syringe
75 mg/0.21 mL administered every one month
One single-dose prefilled syringe
75 mg/0.21 mL administered every one month
Store in refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Allow UZEDY to
UZEDY may be stored in unopened original packaging at room temperature, 20°C to 25°C (68°F to 77°F), for up to 90 days. If unopened, UZEDY may be returned to refrigerated storage within 90 days.
Once the carton is opened, administer UZEDY or discard.
OPEN HERE

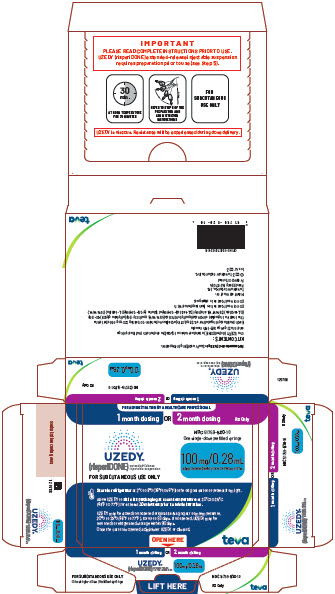
11Package/Label Display Panel
FOR ADMINISTRATION BY A HEALTHCARE PROFESSIONAL
1 month dosing OR 2 month dosing
UZEDY® (risperiDONE) extended-release injectable suspension
FOR SUBCUTANEOUS USE ONLY
FOR SUBCUTANEOUS USE ONLY
NDC 51759-520-10
One single-dose prefilled syringe
100 mg/0.28 mL administered every one or two months
One single-dose prefilled syringe
100 mg/0.28 mL administered every one or two months
Store in refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Allow UZEDY to
UZEDY may be stored in unopened original packaging at room temperature, 20°C to 25°C (68°F to 77°F), for up to 90 days. If unopened, UZEDY may be returned to refrigerated storage within 90 days.
Once the carton is opened, administer UZEDY or discard.
OPEN HERE
12Package/Label Display Panel
1 month dosing
For administration by a healthcare professional
Rx Only
UZEDY® (risperiDONE) extended-release injectable suspension
FOR SUBCUTANEOUS USE ONLY
FOR SUBCUTANEOUS USE ONLY
NDC 51759-630-10
One single-dose prefilled syringe
125 mg/0.35 mL administered every one month
One single-dose prefilled syringe
125 mg/0.35 mL administered every one month
Store in refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Allow UZEDY to
UZEDY may be stored in unopened original packaging at room temperature, 20°C to 25°C (68°F to 77°F), for up to 90 days. If unopened, UZEDY may be returned to refrigerated storage within 90 days.
Once the carton is opened, administer UZEDY or discard.
OPEN HERE

13Package/Label Display Panel
2 month dosing
For administration by a healthcare professional
Rx Only
UZEDY® (risperiDONE) extended-release injectable suspension
FOR SUBCUTANEOUS USE ONLY
FOR SUBCUTANEOUS USE ONLY
NDC 51759-740-10
One single-dose prefilled syringe
150 mg/0.42 mL administered every two months
One single-dose prefilled syringe
150 mg/0.42 mL administered every two months
Store in refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Allow UZEDY to
UZEDY may be stored in unopened original packaging at room temperature, 20°C to 25°C (68°F to 77°F), for up to 90 days. If unopened, UZEDY may be returned to refrigerated storage within 90 days.
Once the carton is opened, administer UZEDY or discard.
OPEN HERE

14Package/Label Display Panel
2 month dosing
For administration by a healthcare professional
Rx Only
UZEDY® (risperiDONE) extended-release injectable suspension
FOR SUBCUTANEOUS USE ONLY
FOR SUBCUTANEOUS USE ONLY
NDC 51759-850-10
One single-dose prefilled syringe
200 mg/0.56 mL administered every two months
One single-dose prefilled syringe
200 mg/0.56 mL administered every two months
Store in refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Allow UZEDY to
UZEDY may be stored in unopened original packaging at room temperature, 20°C to 25°C (68°F to 77°F), for up to 90 days. If unopened, UZEDY may be returned to refrigerated storage within 90 days.
Once the carton is opened, administer UZEDY or discard.
OPEN HERE

15Package/Label Display Panel
2 month dosing
For administration by a healthcare professional
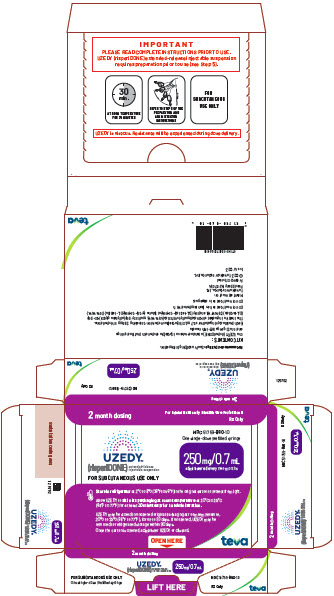
Rx Only
UZEDY® (risperiDONE) extended-release injectable suspension
FOR SUBCUTANEOUS USE ONLY
FOR SUBCUTANEOUS USE ONLY
NDC 51759-960-10
One single-dose prefilled syringe
250 mg/0.7 mL administered every two months
One single-dose prefilled syringe
250 mg/0.7 mL administered every two months
Store in refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Allow UZEDY to
UZEDY may be stored in unopened original packaging at room temperature, 20°C to 25°C (68°F to 77°F), for up to 90 days. If unopened, UZEDY may be returned to refrigerated storage within 90 days.
Once the carton is opened, administer UZEDY or discard.
OPEN HERE