Brand Name
Folotyn
Generic Name
Pralatrexate
View Brand Information FDA approval date: September 24, 2009
Classification: Folate Analog Metabolic Inhibitor
Form: Injection
What is Folotyn (Pralatrexate)?
Pralatrexate injection is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma . This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. Pralatrexate injection is a dihydrofolate reductase inhibitor indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma . This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Folotyn (pralatrexate)
1INDICATIONS AND USAGE
FOLOTYN is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL).
This indication is approved under accelerated approval based on overall response rate
2DOSAGE FORMS AND STRENGTHS
Injection: 40 mg/2 mL (20 mg/mL) and 20 mg/mL clear yellow sterile solution in single-dose vial
3CONTRAINDICATIONS
None
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression
- Mucositis
- Dermatologic Reactions
- Tumor Lysis Syndrome
- Hepatic Toxicity
4.1Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Peripheral T-cell Lymphoma
The safety of FOLOTYN was evaluated in Study PDX-008
Forty-four percent of patients (n = 49) experienced a serious adverse event while on study or within 30 days after their last dose of FOLOTYN. The most common serious adverse events (> 3%), regardless of causality, were pyrexia, mucositis, sepsis, febrile neutropenia, dehydration, dyspnea, and thrombocytopenia. One death from cardiopulmonary arrest in a patient with mucositis and febrile neutropenia was reported in this trial. Across clinical trials, deaths from mucositis, febrile neutropenia, sepsis, and pancytopenia occurred in 1.2% of patients who received doses ranging from 30 mg/m
Twenty-three percent of patients (n = 25) discontinued treatment with FOLOTYN due to adverse reactions. The most frequent adverse reactions reported as the reason for discontinuation of treatment were mucositis (6%) and thrombocytopenia (5%).
The most common adverse reactions (> 35%) were mucositis, thrombocytopenia, nausea, and fatigue.
Table 4 summarizes the adverse reactions in Study PDX-008.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of FOLOTYN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic Reactions: Toxic epidermal necrolysis.
5OVERDOSAGE
No specific information is available on the treatment of overdosage of FOLOTYN. If an overdose occurs, general supportive measures should be instituted as deemed necessary by the treating healthcare provider. Based on FOLOTYN's mechanism of action, consider the prompt administration of leucovorin.
6DESCRIPTION
Pralatrexate is a dihydrofolate reductase inhibitor. Pralatrexate has the chemical name (2S)-2-[[4-[(1RS)-1-[(2, 4-diaminopteridin-6-yl)methyl]but-3- ynyl]benzoyl]amino]pentanedioic acid. The molecular formula is C
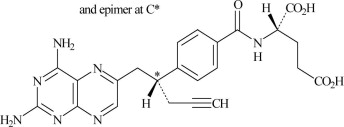
Pralatrexate is an off-white to yellow solid. It is soluble in aqueous solutions at pH 6.5 or higher. Pralatrexate is practically insoluble in chloroform and ethanol. The pKa values are 3.25, 4.76, and 6.17.
FOLOTYN (pralatrexate) is supplied as a preservative-free, sterile, isotonic, non-pyrogenic clear yellow aqueous solution contained in a clear glass single-dose vial (Type I) for intravenous use. Each 1 mL of solution contains 20 mg of pralatrexate, sufficient sodium chloride to achieve an isotonic (280-300 mOsm) solution, and sufficient sodium hydroxide, and hydrochloric acid if needed, to adjust and maintain the pH at 7.5-8.5. FOLOTYN is supplied as either 20 mg (1 mL) or 40 mg (2 mL) single-dose vials at a concentration of 20 mg/mL.
7CLINICAL STUDIES
The efficacy of FOLOTYN was evaluated in Study PDX-008, an open-label, single-arm, multi-center, international trial that enrolled patients with relapsed or refractory PTCL. One hundred and eleven patients received FOLOTYN 30 mg/m
The major efficacy outcome measure was overall response rate (complete response, complete response unconfirmed, and partial response) as assessed by International Workshop Criteria (IWC). An additional efficacy outcome measure was duration of response. Response assessments were scheduled at the end of cycle 1 and then every other cycle (every 14 weeks). Duration of response was measured from the first day of documented response to disease progression or death. Response and disease progression were evaluated by independent central review using the IWC.
Fourteen patients went off treatment in cycle 1; 2 patients were unevaluable for response by IWC due to insufficient materials provided to central review.
The initial response assessment was scheduled at the end of cycle 1. Of the responders, 66% responded within cycle 1. The median time to first response was 45 days (range 37-349 days).
8REFERENCES
1. “OSHA Hazardous Drugs.” OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
9HOW SUPPLIED/STORAGE AND HANDLING
FOLOTYN is available in clear glass single-dose vials containing pralatrexate at a concentration of 20 mg/mL as a preservative-free, sterile, clear yellow solution individually packaged for intravenous use in the following presentations:
NDC 72893-003-01: 20 mg of pralatrexate in 1 mL solution in a vial (20 mg / 1 mL)
NDC 72893-005-01: 40 mg of pralatrexate in 2 mL solution in a vial (40 mg / 2 mL)
Store refrigerated at 2-8°C (36-46°F) [see USP Controlled Cold Temperature] in original carton to protect from light.
10PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Folic Acid and Vitamin B
Advise patients treated with FOLOTYN to take folic acid and vitamin B12 to reduce the risk of possible side effects [.
Advise patients treated with FOLOTYN to take folic acid and vitamin B12 to reduce the risk of possible side effects [.
Myelosuppression
Inform patients of the risk of myelosuppression and to immediately contact their healthcare provider should any signs of infection develop, including fever. Inform patients to contact their healthcare provider if bleeding or symptoms of anemia occur [.
Inform patients of the risk of myelosuppression and to immediately contact their healthcare provider should any signs of infection develop, including fever. Inform patients to contact their healthcare provider if bleeding or symptoms of anemia occur [.
Mucositis
Inform patients of the signs and symptoms of mucositis. Instruct patients on ways to reduce the risk of its development, and on ways to maintain nutrition and control discomfort from mucositis if it occurs [.
Inform patients of the signs and symptoms of mucositis. Instruct patients on ways to reduce the risk of its development, and on ways to maintain nutrition and control discomfort from mucositis if it occurs [.
Dermatologic Reactions
Advise patients about the risks for and the signs and symptoms of dermatologic reactions. Instruct patients to immediately notify their healthcare provider if any skin reactions occur [.
Advise patients about the risks for and the signs and symptoms of dermatologic reactions. Instruct patients to immediately notify their healthcare provider if any skin reactions occur [.
Tumor Lysis Syndrome
Inform patients about the risk of and the signs and symptoms of tumor lysis syndrome. Patients should be instructed to notify their healthcare provider if they experience these symptoms [.
Inform patients about the risk of and the signs and symptoms of tumor lysis syndrome. Patients should be instructed to notify their healthcare provider if they experience these symptoms [.
Concomitant Medications
Patients should be instructed to inform their healthcare provider if they are taking any concomitant medications including prescription drugs (such as trimethoprim/sulfamethoxazole and probenecid) and nonprescription drugs (such as nonsteroidal anti-inflammatory drugs) [.
Patients should be instructed to inform their healthcare provider if they are taking any concomitant medications including prescription drugs (such as trimethoprim/sulfamethoxazole and probenecid) and nonprescription drugs (such as nonsteroidal anti-inflammatory drugs) [.
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females or reproductive potential to inform their healthcare provider of a known or suspected pregnancy [.
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females or reproductive potential to inform their healthcare provider of a known or suspected pregnancy [.
Advise females patients of reproductive potential to use effective contraception during treatment with FOLOTYN and for 6 months after the final dose
Advise males with female partners of reproductive potential to use effective contraception during treatment with FOLOTYN and for at least 3 months after the final dose
Lactation
Advise females women not to breastfeed during treatment with FOLOTYN and for 1 week after the final dose [.
Advise females women not to breastfeed during treatment with FOLOTYN and for 1 week after the final dose [.
Manufactured by:
Manufactured for:
Patents: 6,028,071, 7,622,470 and 8,299,078
11SPL PATIENT PACKAGE INSERT SECTION
Patient Information
FOLOTYN®(FOH-loh-tin)
(pralatrexate injection)
(pralatrexate injection)
What is FOLOTYN?
FOLOTYN is a prescription used to treat people with a type of cancer called peripheral T-cell lymphoma (PTCL) that does not go away, gets worse, or comes back after use of another cancer treatment. It is not known if FOLOTYN is safe and effective in children.
Before you receive FOLOTYN, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems, including end-stage renal disease (ESRD)
- are pregnant or plan to become pregnant. FOLOTYN can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if FOLOTYN passes into your breast milk. Do not breastfeed during treatment with FOLOTYN and for 1 week after the last dose. Talk to your healthcare provider about the best way to feed your baby during treatment with FOLOTYN.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how FOLOTYN works.
Especially tell your healthcare provider if you take:
- sulfamethoxazole trimethoprim
- non-steroidal anti-inflammatory drugs (NSAIDs) medicines
- probenecid
Know the medicines you take. Keep a list of them and show it to your healthcare provider or pharmacist each time you start a new medicine.
How will I receive FOLOTYN?
- FOLOTYN will be given to you by your healthcare provider as an intravenous (IV) injection into your vein over 3 to 5 minutes.
- FOLOTYN is usually given in cycles, one time each week for 6 weeks, with no treatment on the 7th week.
- Your healthcare provider will treat you with folic acid and vitamin B12 before and during your treatment with FOLOTYN to help reduce the risk of possible side effects.
- You will take folic acid by mouth for 10 days before your first dose of FOLOTYN. Continue taking folic acid during treatment with FOLOTYN and for 30 days after the last dose.
- Your healthcare provider will give you a vitamin B12 injection into your muscle (intramuscular). You will get your first vitamin B12 injection 10 weeks before your first dose of FOLOTYN and every 8 to 10 weeks during treatment with FOLOTYN.
- Your healthcare provider will do blood tests before and during treatment with FOLOTYN.
Your healthcare provider may stop treatment, delay treatment, or change your dose of FOLOTYN based on results of your blood tests and if you have certain side effects.
What are the possible side effects of FOLOTYN?
- Low blood cell counts: Your healthcare provider will do blood tests to check your blood cell counts before and during treatment with FOLOTYN. Tell your healthcare provider right away if you develop any signs of infection, fever, bleeding or tiredness during treatment with FOLOTYN.
- Redness and sores of the mucous membrane lining of the mouth, lips, throat, digestive tract, and genitals (mucositis). Mucositis is common with FOLOTYN and can be severe. Tell your healthcare provider if you develop redness or painful sores in your mouth or throat, or have trouble speaking, eating or drinking. Your healthcare provider will tell you about ways to reduce your risk of getting mucositis, and how to maintain nutrition and help control the discomfort from mucositis.
- Severe skin reactions. FOLOTYN can cause severe skin reactions that may lead to death. In people with lymphoma, severe skin reactions may happen on and under your skin. Tell your healthcare provider right away if you develop any f the following skin reactions:
- rash
- peeling and loss of skin
- sores
- blisters
- Tumor Lysis Syndrome (TLS). TLS is caused by the fast breakdown of certain types of cancer cells. Your healthcare provider may do blood tests to check you for TLS and treat you if needed.
- Liver problems. Your healthcare provider will monitor you for liver problems during treatment with FOLOTYN.
- Increased risk of serious reactions in people with kidney problems. People with severe kidney problems may have a greater risk for increased serious reactions during treatment with FOLOTYN.
The most common side effects of FOLOTYN include: low platelet blood counts, nausea, and tiredness.
These are not all of the possible side effects of FOLOTYN.
General information about the safe and effective use of FOLOTYN.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. This Patient Information leaflet summarizes the most important information about FOLOTYN. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about FOLOTYN that is written for health professionals.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. This Patient Information leaflet summarizes the most important information about FOLOTYN. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about FOLOTYN that is written for health professionals.
What are the ingredients in FOLOTYN?
Active ingredient:pralatrexate
Inactive ingredients:sodium chloride, sodium hydroxide, and hydrochloric acid
Manufactured by:
Manufactured for:
Acrotech Biopharma Inc
FOLOTYN and the flower symbol are all registered trademarks of Acrotech Biopharma Inc.
© Acrotech Biopharma Inc. All rights reserved.
For more information, go to www.FOLOTYN.com or call 1-888-255-6788.
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: Aug 2024
12PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE CARTON - FOLOTYN 20 mg/1 mL Vial
NDC 72893-003-01
(pralatrexate injection)
20 mg/mL
For intravenous use
Rx only
1 mL
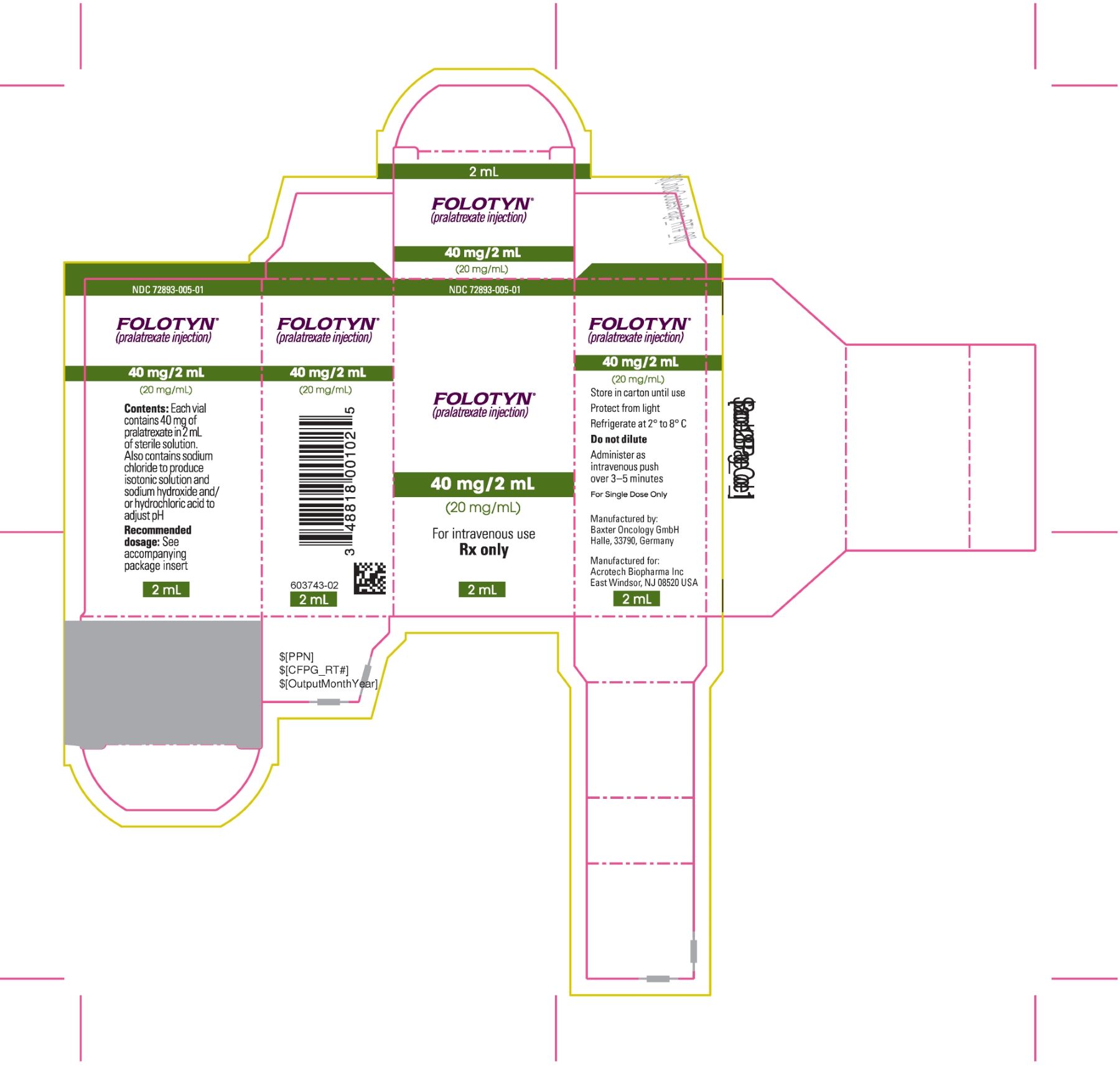
13PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE CARTON - FOLOTYN 40 mg/2 mL Vial
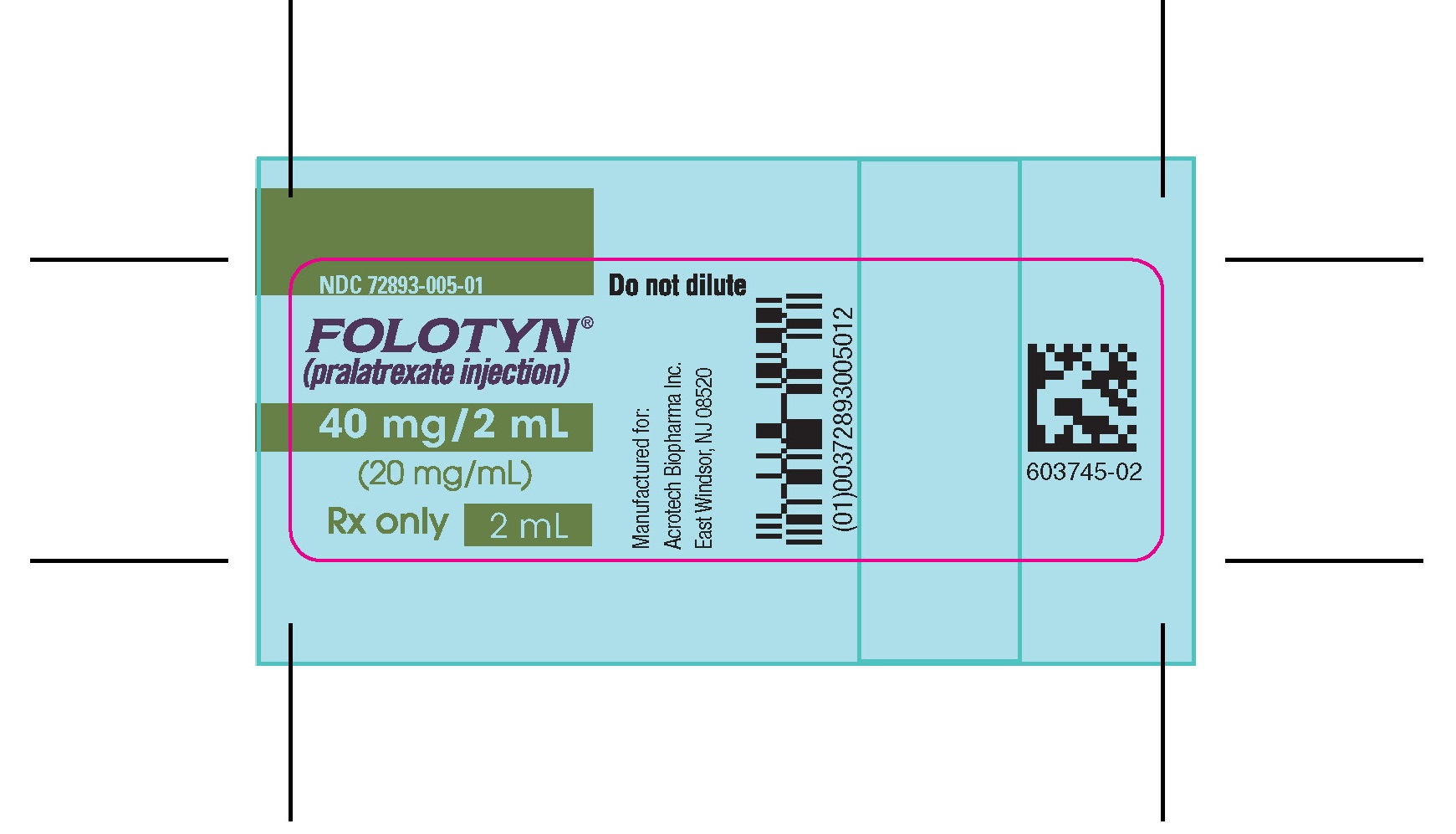
NDC 72893-005-01
FOLOTYN
(pralatrexate injection)
40 mg/2 mL
(20 mg/mL)
For intravenous use
Rx only
2 mL
14PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
VIAL – FOLOTYN 20 mg/1 ml
NDC 72893-003-01
FOLOTYN
(pralatrexate injection)
20 mg/mL
Rx only
1 mL

15PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
VIAL – FOLOTYN 40 mg/2 ml
NDC 72893-005-01
FOLOTYN
(pralatrexate injection)
40 mg/2 mL
Rx only
2 mL