Coartem
What is Coartem (Lumefantrine)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The goal of this open-label randomised, controlled, non-inferiority trial is to assess and compare the efficacy, tolerability and safety of a fixed dose TACT artemether-lumefantrine-amodiaquine (ALAQ) to the ACTs artemether-lumefantrine (AL), artesunate-amodiaquine (ASAQ) (with single low-dose primaquine in some sites) for the treatment of uncomplicated Plasmodium falciparum malaria in patient. Th...
Summary: Platform study to evaluate the efficacy and safety of anti-malarial agents in patients with uncomplicated Plasmodium falciparum malaria
Summary: A prospective study will be carried out in an area where parasites with reduced sensitivity to malaria drugs (artemisinins) have recently emerged. The study will recruit participants from patients who attend the clinic with uncomplicated malaria and asymptomatically infected individuals. Participants are treated with conventional artemisinin-combination therapies (ACT) as part of standard clinical...
Related Latest Advances
Brand Information
- Coartem Tablets are not approved for patients with severe or complicated
- Coartem Tablets are not approved for the prevention of malaria.
- Hypersensitivity Reactions
- Prolongation of the QT Interval
- Use of QT Prolonging Drugs and Other Antimalarials
- Drug Interactions with CYP3A4
- Drug Interactions with CYP2D6
- Hypersensitivity Reactions: anaphylaxis, urticaria, angioedema, and serious skin reactions (bullous eruption) have been reported.
- Blood and Lymphatic System Disorders: Cases of delayed hemolytic anemia have been reported following treatment with artemether-lumefantrine, mostly when used for treatment of severe malaria in patients initially treated with IV/parenteral artesunate. Coartem Tablets should not be used to treat severe malaria as it is not an approved indication.
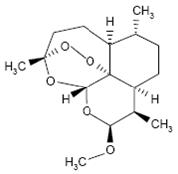
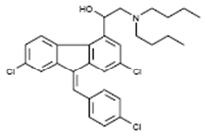
- Instruct patients to inform their physician of any personal or family history of QT prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia
- Instruct patients to inform their physician if they are taking any other medications that prolong the QT interval, such as Class IA (quinidine, procainamide, disopyramide), or Class III (amiodarone, sotalol) antiarrhythmic agents; antipsychotics (pimozide, ziprasidone); antidepressants; certain antibiotics (macrolide antibiotics, fluoroquinolone antibiotics, imidazole, and triazole antifungal agents)
- Instruct patients to notify their physicians if they have any symptoms of prolongation of the QT interval, including prolonged heart palpitations or a loss of consciousness