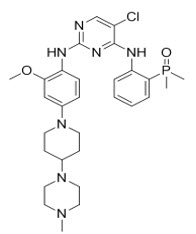
Alunbrig
What is Alunbrig (Brigatinib)?
Approved To Treat
Related Clinical Trials
Summary: Targeted cancer therapies have a higher therapeutic index than chemotherapy and are prescribed to tens of thousands of patients in France each year. These treatments modify often ubiquitous signaling pathways involved in neuronal synaptic plasticity, the cellular substrate of cognitive and psychiatric functions. Neurocognitive and psychiatric disorders associated with targeted therapies are poorly...
Summary: This is an open-label, phase I-II dose-escalation and expansion study designed to define the recommended dose of brigatinib as monotherapy in pediatric and young adult patients with ALK+ ALCL, IMT or other solid tumors and to evaluate the pharmacokinetics (PK), (long-term) safety, and efficacy of brigatinib in these children.
Summary: This study is a survey in Japan of Brigatinib tablets used to treat Japanese people with non-small cell lung cancer. The study sponsor will not be involved in how the participants are treated but will provide instructions on how the clinics will record what happens during the study. The main aim of the study is to check for side effects related to lung disease from Brigatinib. During the study, pa...
Related Latest Advances
Brand Information
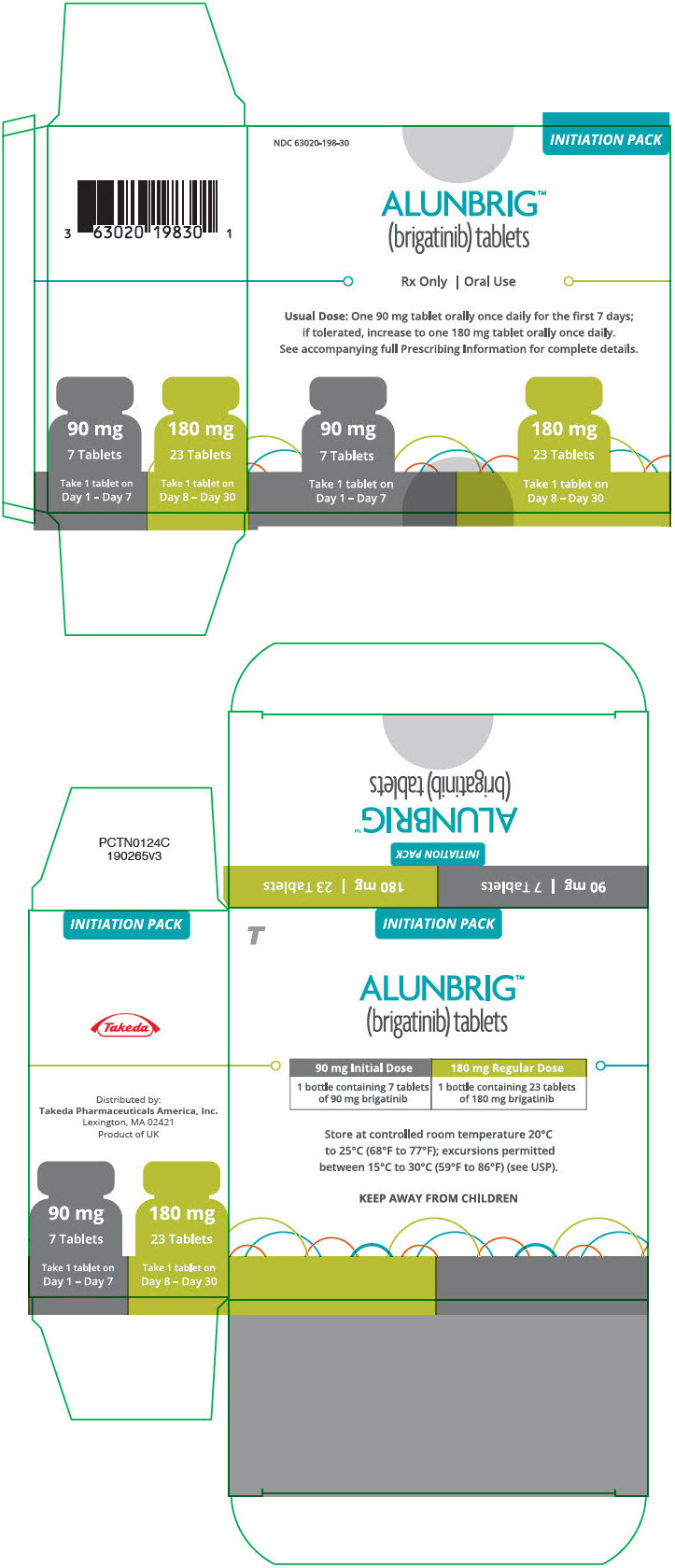
- 180 mg: oval, white to off-white film-coated tablets with "U13" debossed on one side and plain on the other side
- 90 mg: oval, white to off-white film-coated tablets with "U7" debossed on one side and plain on the other side
- 30 mg: round, white to off-white film-coated tablets with "U3" debossed on one side and plain on the other side
- Interstitial Lung Disease (ILD)/Pneumonitis
- Hypertension
- Bradycardia
- Visual Disturbance
- Creatine Phosphokinase (CPK) Elevation
- Pancreatic Enzymes Elevation
- Hepatotoxicity
- Hyperglycemia
- Photosensitivity
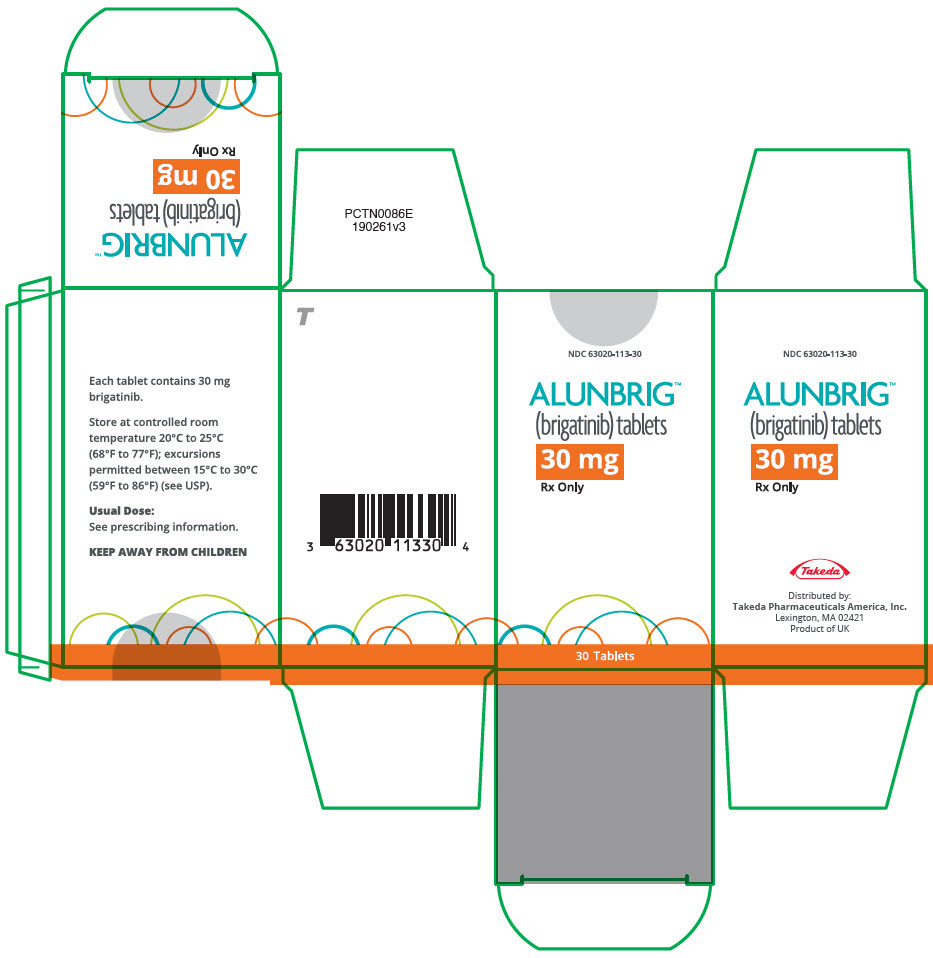
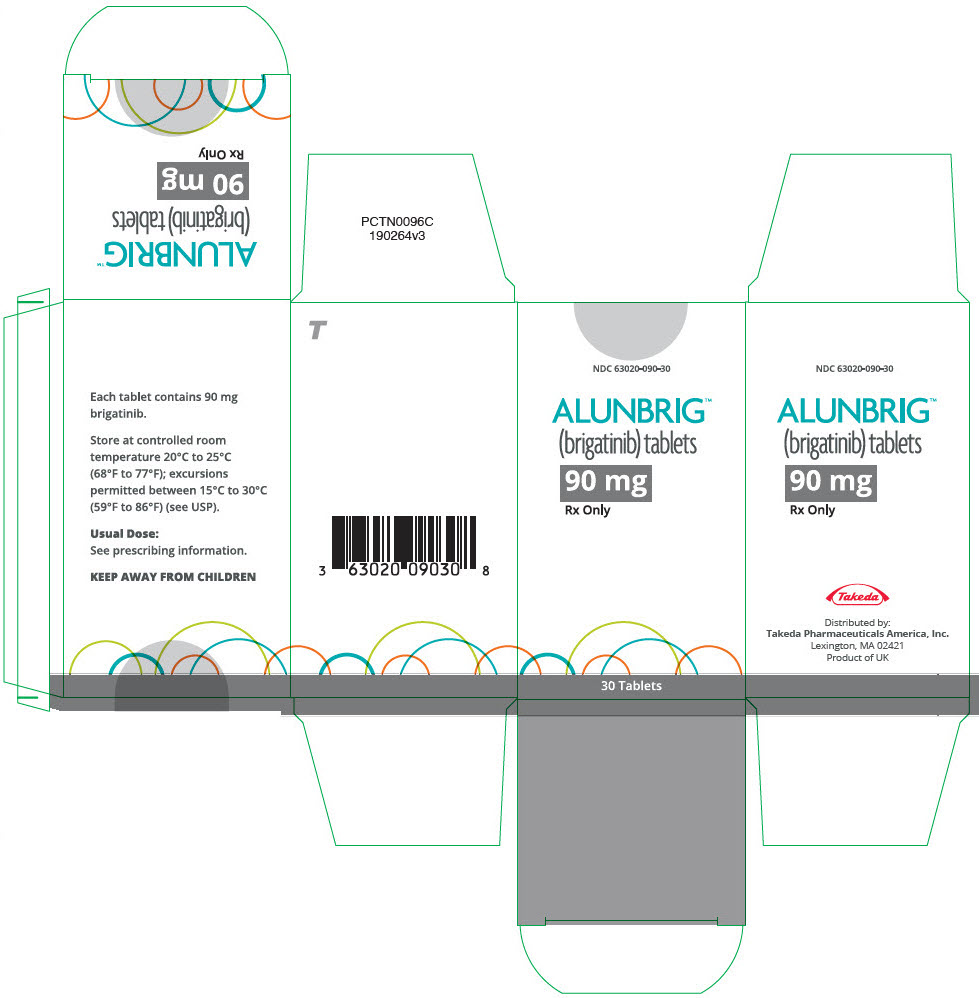
INITIATION PACK