Enspryng
What is Enspryng (Satralizumab)?
Approved To Treat
Related Clinical Trials
Summary: The purpose of this study is to assess the efficacy, safety, PK, and PD of satralizumab in participants with NMDAR and LGI1 encephalitis.
Summary: The purpose of this study is to assess the efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) of satralizumab, a humanized anti-interleukin-6 receptor (aIL-6R) monoclonal antibody, in ambulatory and non-ambulatory participants with DMD age ≥ 8 to \< 18 years old receiving corticosteroid therapy.
Summary: This study will primarily evaluate the pharmacokinetics of satralizumab in pediatric patients aged 2-11 years with anti-aquaporin-4 (AQP4) antibody seropositive neuromyelitis optica spectrum disorder (NMOSD). Efficacy, safety, tolerability, and pharmacodynamics will be evaluated in a descriptive manner, given the small number of patients who will be enrolled in this study.
Related Latest Advances
Brand Information
- A known hypersensitivity to satralizumab or any of the inactive ingredients
- Active Hepatitis B infection
- Active or untreated latent tuberculosis
- Infections
- Elevated Liver Enzymes
- Decreased Neutrophil Counts
- Hypersensitivity Reactions
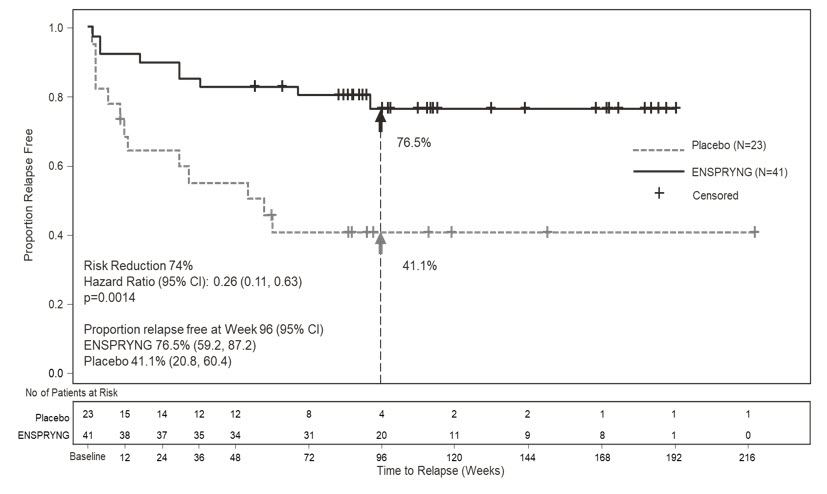
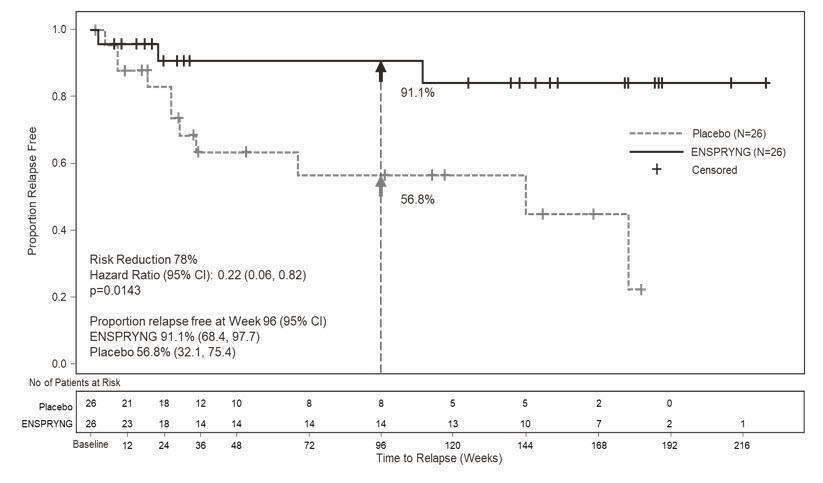
- Study 1: Clinical evidence of 1 relapse in the previous 12 months
- Study 2: Clinical evidence of at least 2 relapses in the previous 2 years, at least one of which must have occurred in the previous year
- EDSS score of 0 to 6.5 (both studies)
- Study 1: Patients were excluded if previously treated with IST within an interval specified for each such therapy
- Study 2: One of the following baseline treatments at a stable dose as a monotherapy for 8 weeks prior to baseline: azathioprine, mycophenolate mofetil, oral corticosteroids
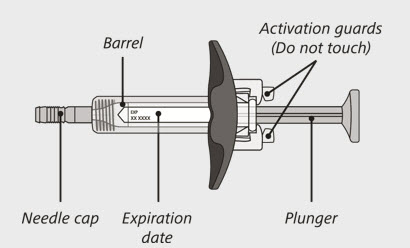
- Each syringe is prefilled with a medicine called ENSPRYNG.
- Each carton of ENSPRYNG contains only 1 prefilled syringe.
- Each prefilled syringe can be used only 1 time.
- take the needle cap off until you are ready to inject ENSPRYNG.
- use the syringe if it has been dropped or damaged.
- try to take the syringe apart at any time.
- leave the syringe unattended.
- re-use the same syringe.
- Keep the unused syringe in the refrigerator between
- Before giving an injection, if the ENSPRYNG is not opened, it can be removed from and placed back in the refrigerator if needed. The total combined time out of the refrigerator should not be more than 8 days at a temperature that does not go above 86°F (30°C).
- Keep the syringe in its original carton away from direct sunlight.
- Always keep the syringe dry.
- freeze the syringe.
- use the syringe if it has been frozen.
- shake.
- 1 prefilled syringe for 1-time use only.
- 1 alcohol pad
- 1 sterile cotton ball or gauze
- 1 small bandage
- 1 FDA-cleared puncture-resistant sharps container for safe disposal of the needle cap and used syringe. See
- Take the carton containing the syringe out of the refrigerator and place it on a clean, flat work surface (like a table).
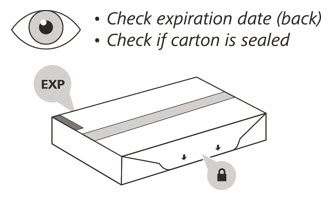
- Check the expiration (EXP) date on the back of the carton
- Check the front of the carton to make sure it is sealed
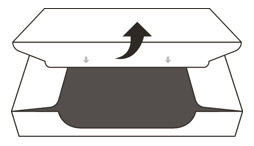
- Open the sealed carton
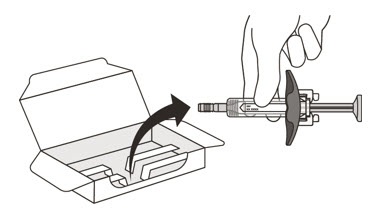
- Carefully lift the syringe out of the carton by holding the barrel
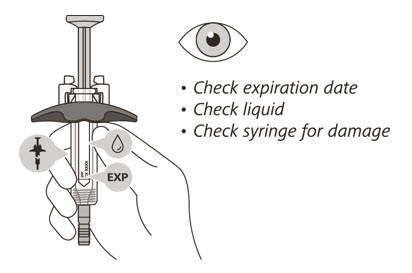
- Check the expiration date on the syringe.
- Check the syringe for any damage.
- Check that the liquid through the viewing window is clear and colorless to slightly yellow.
- After you have checked the syringe, place it on a clean, flat work surface (like a table) for
- Wash your hands with soap and water.
- Choose your injection site in either:
- Wipe the injection site with an alcohol pad and let it air dry.
- Hold the barrel of the syringe between your thumb and index finger. With your other hand, pull the needle cap straight off. You may see a drop of liquid at the end of the needle. This is normal and will not affect your dose
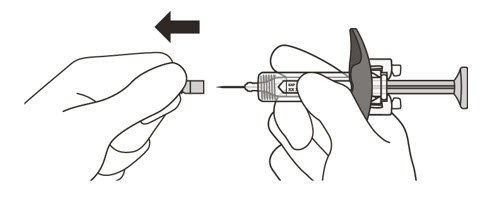
- Throw away the needle cap in a puncture-resistant sharps container immediately. See
- Hold the barrel of the syringe using your thumb and index finger. With your other hand, pinch the area of skin you have cleaned
- Use a quick, dart-like motion to insert the needle at an angle between 45° to 90°
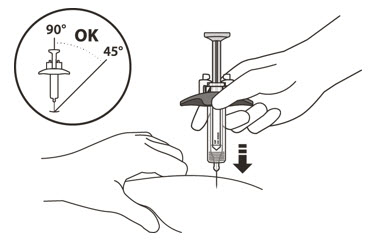
- After the needle is inserted, let go of the pinched skin.
- Slowly inject all of the medicine by gently pushing the plunger all the way down until it touches the activation guards
- Gently release the plunger and allow the needle to come out of the skin at the same angle it was inserted
- There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site but
- Put your used syringe in an FDA-cleared sharps disposal container immediately after use
