Brand Name
Cresemba
Generic Name
Isavuconazonium
View Brand Information FDA approval date: March 06, 2015
Classification: Azole Antifungal
Form: Injection, Capsule
What is Cresemba (Isavuconazonium)?
CRESEMBA ® is an azole antifungal indicated for use in the treatment of: Invasive aspergillosis.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Phase 1 Study of Ibrutinib and Immuno-Chemotherapy Using Temozolomide, Etoposide, Doxil, Dexamethasone, Ibrutinib, Rituximab (TEDDI-R) in Primary CNS Lymphoma
Summary: BACKGROUND: * Primary CNS lymphoma (PCNSL) is a rare subtype of diffuse large B-cell lymphoma. * The outcome for patients with this diagnosis is significantly worse than for that of systemic DLBCL. Most treatment approaches in the past have included high dose methotrexate and radiation treatment. * Most PCNSLs appear to be of activated B-cell (ABC) origin. * Ibrutinib is an inhibitor of Bruton s t...
A Prospective, Single-arm, Open-label, Non-interventional, Multi-centre, Post Marketing Surveillance (PMS) Study of Cresemba to Evaluate Safety and Effectiveness in Patients With Invasive Aspergillosis or Invasive Mucormycosis in Korea
Summary: The purpose of this study is to observe safety and effectiveness of Cresemba in patients with invasive Aspergillosis or invasive Mucormycosis in Korea during the post-marketing surveillance period as required by Ministry of Food and Drug Safety (MFDS).
Related Latest Advances
Brand Information
CRESEMBA (isavuconazonium sulfate)
1DOSAGE FORMS AND STRENGTHS
CRESEMBA Capsules
CRESEMBA 74.5 mg isavuconazonium sulfate (equivalent to 40 mg of isavuconazole) capsules are opaque and have a Swedish orange (reddish-brown) body imprinted with the Astellas logo in black ink and a Swedish orange cap imprinted with “557” in black ink.
CRESEMBA 186 mg isavuconazonium sulfate (equivalent to 100 mg of isavuconazole) capsules are opaque, elongated and have a Swedish orange (reddish-brown) body imprinted with the Astellas logo in black ink and a white cap imprinted with “766” in black ink.
CRESEMBA for Injection
Each single-dose vial of CRESEMBA for injection contains 372 mg isavuconazonium sulfate (equivalent to 200 mg of isavuconazole). CRESEMBA for injection is supplied in a single-dose vial as a sterile lyophilized white to yellow powder.
2CONTRAINDICATIONS
- CRESEMBA is contraindicated in persons with known hypersensitivity to isavuconazole.
- Coadministration of strong CYP3A4 inhibitors, such as ketoconazole or high-dose ritonavir (400 mg every 12 hours), with CRESEMBA is contraindicated because strong CYP3A4 inhibitors can significantly increase the plasma concentration of isavuconazole
- Coadministration of strong CYP3A4 inducers, such as rifampin, carbamazepine, St. John’s wort, or long acting barbiturates with CRESEMBA is contraindicated because strong CYP3A4 inducers can significantly decrease the plasma concentration of isavuconazole
- CRESEMBA shortened the QTc interval in a concentration-related manner. CRESEMBA is contraindicated in patients with familial short QT syndrome
3ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Hepatic Adverse Drug Reactions
- Infusion-Related Reactions
- Hypersensitivity Reactions
- Embryo-Fetal Toxicity
3.1Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of CRESEMBA cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials Experience in Adult Patients
A total of 403 adult patients were exposed to CRESEMBA in two clinical trials. The most frequently reported adverse reactions among CRESEMBA-treated patients were nausea (26%), vomiting (25%), diarrhea (22%), headache (17%), elevated liver chemistry tests (16%), hypokalemia (14%), constipation (13%), dyspnea (12%), cough (12%), peripheral edema (11%), and back pain (10%). Serious adverse reactions occurred in 223/403 (55%) of patients and 56/403 (14%) of patients permanently discontinued treatment with CRESEMBA due to an adverse reaction in the two trials. The adverse reactions which most often led to permanent discontinuation of CRESEMBA therapy during the clinical trials were confusional state (0.7%), acute renal failure (0.7%), increased blood bilirubin (0.5%), convulsion (0.5%), dyspnea (0.5%), epilepsy (0.5%), respiratory failure (0.5%), and vomiting (0.5%).
Patients in the clinical trials were immunocompromised with underlying conditions including hematological malignancy, neutropenia post-chemotherapy, graft-versus-host disease, and hematopoietic stem cell transplant. The patient population was 61% male, had a mean age of 51 years (range 17-92, including 85 patients aged greater than 65 years), and was 79% White and 3% Black. One hundred forty-four (144) patients had a duration of CRESEMBA therapy of greater than 12 weeks, with 52 patients receiving CRESEMBA for over six months.
In Trial 1, a randomized, double-blind, active-controlled clinical trial for treatment of invasive aspergillosis, treatment‑emergent adverse reactions occurred in 247/257 (96%), and 255/259 (99%) patients in the CRESEMBA and voriconazole treatment groups, respectively. Adverse reactions resulting in permanent discontinuation were reported in 37 (14%) CRESEMBA-treated patients and 59 (23%) voriconazole-treated patients.
In Trial 2, an open-label, non-comparative trial of CRESEMBA in patients with invasive aspergillosis and renal impairment or invasive mucormycosis, adverse reactions occurred in 139/146 (95%) of patients in the CRESEMBA treatment group. Adverse reactions resulting in permanent discontinuation were reported in 19 (13%) CRESEMBA‑treated patients. The frequencies and types of adverse reactions observed in CRESEMBA-treated patients were similar between Trial 1 and Trial 2.
The following adverse reactions occurred in less than 5% of all CRESEMBA-treated patients in Trial 1 or 2. The list does not include reactions presented in
- Blood and lymphatic system disorders: agranulocytosis, leukopenia, pancytopenia
- Cardiac disorders: atrial fibrillation, atrial flutter, bradycardia, reduced QT interval on electrocardiogram, palpitations, supraventricular extrasystoles, supraventricular tachycardia, ventricular extrasystoles, cardiac arrest
- Ear and labyrinth disorders: tinnitus, vertigo
- Eye disorders: optic neuropathy
- Gastrointestinal disorders: abdominal distension, gastritis, gingivitis, stomatitis
- General disorders and administration site conditions: catheter thrombosis, malaise, chills
- Hepatobiliary disorders: cholecystitis, cholelithiasis, hepatitis, hepatomegaly, hepatic failure
- Immune system disorders: hypersensitivity
- Injury, poisoning and procedural complications: fall
- Metabolism and nutrition disorders: hypoalbuminemia, hypoglycemia, hyponatremia
- Musculoskeletal and connective tissue disorders: myositis, bone pain, neck pain
- Nervous system disorders: convulsion, dysgeusia, encephalopathy, hypoesthesia, migraine, peripheral neuropathy, paresthesia, somnolence, stupor, syncope, tremor
- Psychiatric disorders: confusion, hallucination, depression
- Renal and urinary disorders: hematuria, proteinuria
- Respiratory, thoracic and mediastinal disorders: bronchospasm, tachypnea
- Skin and subcutaneous tissue disorders: alopecia, dermatitis, exfoliative dermatitis, erythema, petechiae, urticaria
- Vascular disorders: thrombophlebitis
Laboratory Effects
In Trial 1, elevated liver transaminases (alanine aminotransferase or aspartate aminotransferase) greater than three times the upper limit of normal were reported at the end of study treatment in 4.4% of patients who received CRESEMBA. Elevations of liver transaminases greater than ten times the upper limit of normal developed in 1.2% of patients who received CRESEMBA.
In Trial 1, elevated liver transaminases (alanine aminotransferase or aspartate aminotransferase) greater than three times the upper limit of normal were reported at the end of study treatment in 4.4% of patients who received CRESEMBA. Elevations of liver transaminases greater than ten times the upper limit of normal developed in 1.2% of patients who received CRESEMBA.
Clinical Trials Experience in Pediatric Patients
The clinical safety of CRESEMBA was assessed in 77 pediatric patients who received at least one dose of intravenous or oral CRESEMBA in two uncontrolled studies. Fifteen (19.5%) subjects were in the 1 to < 6 years old cohort, 30 subjects (39.0%) were in the 6 to < 12 years old cohort, and 32 subjects (41.6%) were in the 12 to < 18 years old cohort. The duration of treatment ranged from 1 to 181 days with a median duration of treatment of 15 days. The most frequently reported adverse reactions were diarrhea (26%), abdominal pain (23%), vomiting (21%), elevated liver chemistry tests (18%), rash (14%), nausea (13%), pruritus (13%) and headache (12%). In general, adverse reactions (including serious adverse reactions and adverse reactions leading to permanent discontinuation of CRESEMBA) were similar to those reported in adults.
3.2Post-Marketing Experience
The following additional adverse reactions have been identified during post-approval use of CRESEMBA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Immune system disorders: anaphylactic reaction
4DRUG INTERACTIONS
Isavuconazole is a sensitive substrate of CYP3A4. CYP3A4 inhibitors or inducers may alter the plasma concentrations of isavuconazole.
Isavuconazole is a moderate inhibitor of CYP3A4, and a mild inhibitor of P-glycoprotein (P-gp), and organic cation transporter 2 (OCT2).
Drug interaction studies were conducted to investigate the effect of coadministered drugs on the pharmacokinetics of isavuconazole and the effect of isavuconazole on the pharmacokinetics of coadministered drugs
5OVERDOSAGE
During clinical studies, total daily CRESEMBA doses higher than the recommended dose regimen were associated with an increased rate of adverse reactions. At supratherapeutic doses (three times the recommended maintenance dose) evaluated in a thorough QT study, there were proportionally more treatment-emergent adverse reactions than in the therapeutic dose group (maintenance dose) for the following: headache, dizziness, paresthesia, somnolence, disturbance in attention, dysgeusia, dry mouth, diarrhea, oral hypoesthesia, vomiting, hot flush, anxiety, restlessness, palpitations, tachycardia, photophobia and arthralgia. Adverse reactions leading to discontinuation of study drug occurred in 7 of 39 (17.9%) subjects in the supratherapeutic dose group.
Isavuconazole is not removed by hemodialysis. There is no specific antidote for isavuconazole. Treatment should be supportive with appropriate monitoring.
6DESCRIPTION
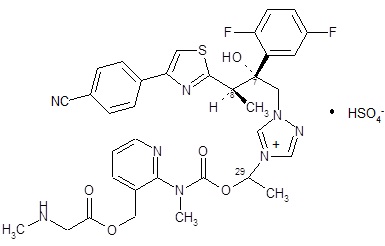
CRESEMBA contains isavuconazonium sulfate, which is the prodrug of isavuconazole, an azole antifungal drug. Isavuconazonium sulfate drug substance is an amorphous, white to yellowish-white powder. The chemical name of isavuconazonium sulfate is glycine,
CRESEMBA Capsules
CRESEMBA (isavuconazonium sulfate) 74.5 mg capsules are available for oral administration. Each CRESEMBA capsule contains 74.5 mg isavuconazonium sulfate, equivalent to 40 mg isavuconazole. The inactive ingredients include black iron oxide, colloidal silicon dioxide, disodium edetate, gellan gum, hypromellose, magnesium citrate, microcrystalline cellulose, potassium acetate, potassium hydroxide, propylene glycol, purified water, red iron oxide, shellac, sodium lauryl sulfate, stearic acid, strong ammonia solution, talc and titanium dioxide.
CRESEMBA (isavuconazonium sulfate) 186 mg capsules are available for oral administration. Each CRESEMBA capsule contains 186 mg isavuconazonium sulfate, equivalent to 100 mg isavuconazole. The inactive ingredients include black iron oxide, colloidal silicon dioxide, disodium edetate, gellan gum, hypromellose, magnesium citrate, microcrystalline cellulose, potassium acetate, potassium hydroxide, propylene glycol, purified water, red iron oxide, shellac, sodium lauryl sulfate, stearic acid, strong ammonia solution, talc and titanium dioxide.
CRESEMBA for Injection
CRESEMBA (isavuconazonium sulfate) for injection is available for intravenous administration. CRESEMBA for injection is a white to yellow sterile, lyophilized powder containing 372 mg isavuconazonium sulfate, equivalent to 200 mg isavuconazole, per vial. Inactive ingredients included in each vial are 96 mg mannitol and sulfuric acid for pH adjustment.
7REFERENCES
- DePauw, B., Walsh, T.J., Donnelly, J.P.,
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Important Administration Instructions
Advise patients that CRESEMBA can be taken with or without food. Each capsule should be swallowed whole. Do not chew, crush, dissolve, or open the capsules
Drug Interactions
Advise patients to inform their physician if they are taking other drugs or before they begin taking other drugs as certain drugs can decrease or increase the plasma concentrations of CRESEMBA
CRESEMBA can decrease or increase the plasma concentrations of other drugs
Pregnancy
Advise patients to inform their physician if they are pregnant, plan to become pregnant, or are nursing
Allergic Reactions
Advise patients to inform their physician immediately if they have ever had an allergic reaction to isavuconazole or other antifungal medicines such as ketoconazole, fluconazole, itraconazole, posaconazole, or voriconazole. Advise patients to discontinue CRESEMBA and seek immediate medical attention if any signs or symptoms of severe allergic reaction occur
Licensed from: Basilea Pharmaceutica International Ltd.
9Package/Label Display Panel – CRESEMBA capsules 186 mg – blister carton label

NDC 0469-0520-02
CRESEMBA
186 mg*
*equivalent to 100 mg isavuconazole
Unit dose blister
Rx Only
10Package/Label Display Panel - CRESEMBA for injection 372 mg – individual vial carton label

NDC 0469-0420-01
CRESEMBA
372 mg*
*equivalent to 200 mg isavuconazole
For Intravenous Infusion Only
Single Dose Vial
Discard unused portion
Rx Only
11Package/Label Display Panel – CRESEMBA capsules 74.5 mg – blister carton label

NDC 0469-2860-35
CRESEMBA
74.5 mg*
*equivalent to 40 mg isavuconazole
Unit dose blister
Rx Only



