Brand Name
Viltepso
Generic Name
Viltolarsen
View Brand Information FDA approval date: August 13, 2020
Form: Injection
What is Viltepso (Viltolarsen)?
VILTEPSO is indicated for the treatment of Duchenne muscular dystrophy in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping. This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with VILTEPSO. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. VILTEPSO is an antisense oligonucleotide indicated for the treatment of Duchenne muscular dystrophy in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping. This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with VILTEPSO. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Viltepso (viltolarsen)
1INDICATIONS AND USAGE
VILTEPSO is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping. This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with VILTEPSO
2DOSAGE FORMS AND STRENGTHS
VILTEPSO is a clear and colorless solution available as follows:
- Injection: 250 mg/5 mL (50 mg/mL) solution in a single-dose vial
3CONTRAINDICATIONS
None.
4DESCRIPTION
VILTEPSO (viltolarsen) injection is a sterile, preservative-free, aqueous solution for intravenous administration. VILTEPSO is a clear and colorless solution. VILTEPSO is supplied in single-dose vials containing 250 mg/5 mL viltolarsen (50 mg/mL) in 0.9% sodium chloride. Each milliliter of VILTEPSO contains 50 mg viltolarsen and 9 mg sodium chloride in water for injection. The final product is adjusted to a pH ranging between 7.0 and 7.5 using hydrochloric acid and/or sodium hydroxide.
Viltolarsen is an antisense oligonucleotide of the phosphorodiamidate morpholino oligomer (PMO) subclass. PMOs are synthetic molecules in which the five-membered ribofuranosyl rings found in natural DNA and RNA are replaced by a six-membered morpholino ring. Each morpholino ring is linked through an uncharged phosphorodiamidate moiety rather than the negatively charged phosphate linkage that is present in natural DNA and RNA. Each phosphorodiamidate morpholino subunit contains one of the heterocyclic bases found in DNA (adenine, cytosine, guanine, or thymine). Viltolarsen contains 21 linked subunits. The molecular formula of viltolarsen is C
Figure 1: Structural Formula of Viltolarsen
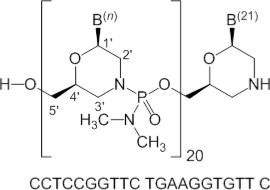
5CLINICAL STUDIES
The effect of VILTEPSO on dystrophin production was evaluated in one study in DMD patients with a confirmed mutation of the DMD gene that is amenable to exon 53 skipping (Study 1; NCT02740972).
Study 1 was a multicenter, 2-period, dose-finding study conducted in the United States and Canada.
During the initial period (first 4 weeks) of Study 1, patients were randomized (double blind) to VILTEPSO or placebo. All patients then received 20 weeks of open-label VILTEPSO 40 mg/kg once weekly (0.5 times the recommended dosage) (N=8) or 80 mg/kg once weekly (N=8). Study 1 enrolled ambulatory male patients 4 years to less than 10 years of age (median age 7 years) on a stable corticosteroid regimen for at least 3 months.
Efficacy was assessed based on change from baseline in dystrophin protein level (measured as % of the dystrophin level in healthy subjects, i.e., % of normal) at Week 25. Muscle biopsies (left or right biceps brachii) were collected from patients at baseline and following 24 weeks of VILTEPSO treatment, and analyzed for dystrophin protein level by Western blot normalized to myosin heavy chain (primary endpoint) and mass spectrometry (secondary endpoint).
In patients who received VILTEPSO 80 mg/kg once weekly, mean dystrophin levels increased from 0.6% (SD 0.8) of normal at baseline to 5.9% (SD 4.5) of normal by Week 25, with a mean change in dystrophin of 5.3% (SD 4.5) of normal levels (p=0.01) as assessed by validated Western blot (normalized to myosin heavy chain); the median change from baseline was 3.8%. All patients demonstrated an increase in dystrophin levels over their baseline values. As assessed by mass spectrometry (normalized to filamin C), mean dystrophin levels increased from 0.6% (SD 0.2) of normal at baseline to 4.2% (SD 3.7) of normal by Week 25, with a mean change in dystrophin of 3.7% (SD 3.8) of normal levels (nominal p=0.03, not adjusted for multiple comparisons); the median change from baseline was 1.9%.
Individual patient dystrophin levels in patients evaluated in Study 1 are shown in
Figure 2: Dystrophin Expression in Individual Patients (Study 1)
Patients Treated With VILTEPSO 80 mg/kg/week (n=8)
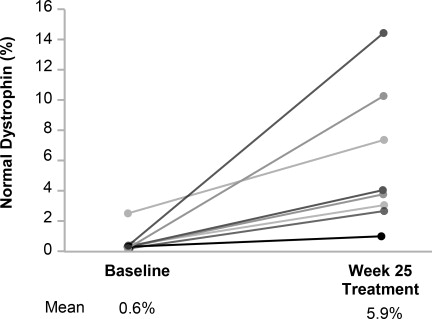
Note: Solid lines represent individual patient data. Dystrophin was measured using Western blot and normalized to myosin heavy chain.
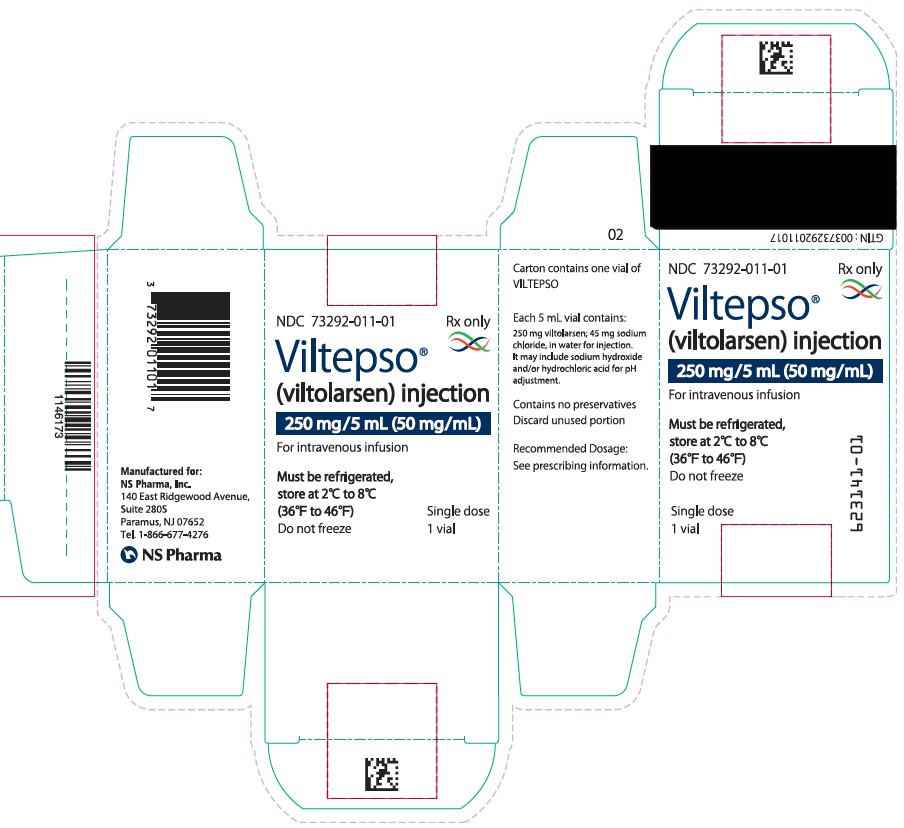
6Principal Display Panel – Carton Label
NDC 73292-011-01
Rx only
Viltepso
(viltolarsen) injection
250 mg/5 mL (50 mg/mL)
For intravenous infusion
Must be refrigerated,
(36°F to 46°F)
Do not freeze
Single dose
1 vial
7Principal Display Panel – Vial Label
NDC 73292-011-01
Viltepso
(viltolarsen) injection
250 mg/5 mL (50 mg/mL)
For intravenous infusion
Refrigerate at 2°C to 8°C (36°F to 46°F)
Mfg for: NS Pharma, Inc.
Rx only
