Brand Name
Soaanz
Generic Name
Torsemide
View Brand Information FDA approval date: June 01, 2004
Classification: Loop Diuretic
Form: Tablet
What is Soaanz (Torsemide)?
SOAANZ is indicated in adults for the treatment of edema associated with heart failure or renal disease. SOAANZ is a loop diuretic indicated in adults for the treatment of edema associated with heart failure or renal disease.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
SOAANZ (Torsemide)
1INDICATIONS AND USAGE
SOAANZ is indicated in adults for the treatment of edema associated with heart failure or renal disease.
2DOSAGE AND ADMINISTRATION
The recommended initial dose is 20 mg oral SOAANZ once daily. If the diuretic response is inadequate, titrate upward by approximately doubling until the desired diuretic response is obtained. Doses higher than 200 mg have not been adequately studied.
3CONTRAINDICATIONS
SOAANZ is contraindicated in patients with known hypersensitivity to SOAANZ.
SOAANZ is contraindicated in patients who are anuric.
SOAANZ is contraindicated in patients with hepatic coma.
4ADVERSE REACTIONS
The following risks are discussed in more detail in other sections:
- Hypotension and Worsening Renal Function
- Electrolyte and Metabolic Abnormalities
- Ototoxicity
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In pre-approval studies, SOAANZ has been evaluated for safety in 65 subjects. Discontinuation of therapy due to adverse reactions occurred in 4 out of the 65 of subjects (6%) treated with SOAANZ.
4.2Postmarketing Experience
The following adverse reactions have been identified during the post-approval use of torsemide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or establish a causal relationship to drug exposure.
Gastrointestinal System: Pancreatitis, abdominal pain
Nervous System: Paresthesia, confusion, visual impairment, loss of appetite
Hematologic: Leucopenia, thrombocytopenia, anemia
Hepatobiliary: Increase in liver transaminases, gamma-glutamyltransferase
Metabolism: Thiamine (vitamin B1) deficiency
Skin/hypersensitivity: Stevens-Johnson syndrome, toxic epidermal necrolysis, photosensitivity reaction, pruritus
Urogenital: Acute urinary retention
5OVERDOSAGE
The signs and symptoms of overdosage can be anticipated to include those of excessive pharmacologic effect: dehydration, hypovolemia, hypotension, hyponatremia, hypokalemia, hypochloremic alkalosis, and hemoconcentration. Treatment of overdosage should consist of fluid and electrolyte replacement.
Laboratory determinations of serum levels of torsemide and its metabolites are not widely available.
No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of torsemide and its metabolites. Torsemide is not dialyzable, so hemodialysis will not accelerate elimination.
6DESCRIPTION
SOAANZ contains torsemide, a diuretic of the pyridine-sulfonylurea class. Its chemical name is 1-isopropyl-3-[(4-m-toluidino-3-pyridyl) sulfonyl] urea and its structural formula is:
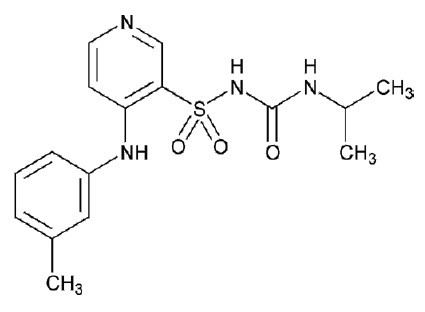
Its empirical formula is C
Torsemide is a white to off-white crystalline powder. The tablets for oral administration also contain colloidal silicon dioxide, hypromellose, iron oxide yellow, iron oxide red, lactose, magnesium stearate, microcrystalline cellulose, talc, polyethylene glycol and titanium dioxide.
7HOW SUPPLIED/STORAGE AND HANDLING
SOAANZ tablets are available as round, film-coated, debossed on one side in the following configurations:
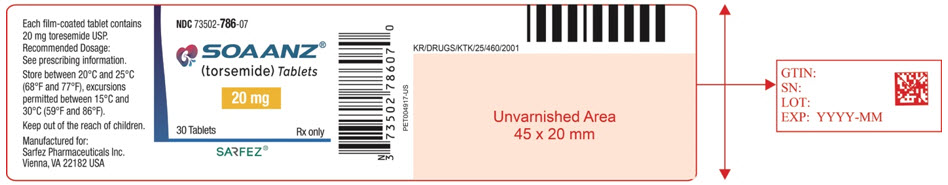
8PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label - 786-07
NDC 73502-786-07
SOAANZ
20 mg
30 Tablets
SARFEZ
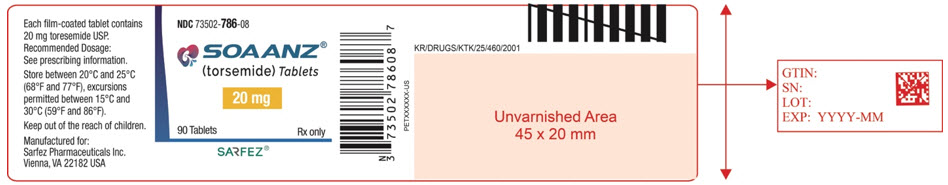
9PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label - 786-08
NDC 73502-786-08
SOAANZ
20 mg
90 Tablets
SARFEZ
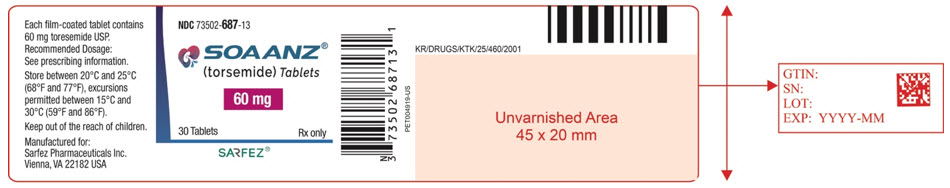
10PRINCIPAL DISPLAY PANEL - 60 mg Tablet Bottle Label - 687-13
NDC 73502-687-13
SOAANZ
60 mg
30 Tablets
SARFEZ
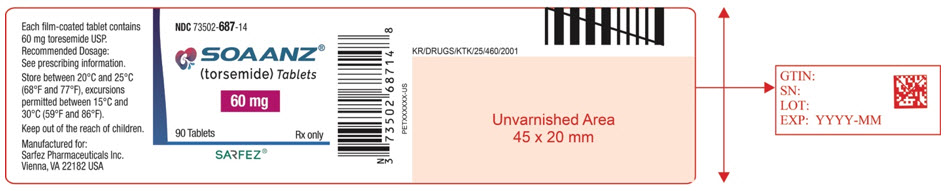
11PRINCIPAL DISPLAY PANEL - 60 mg Tablet Bottle Label - 687-14
NDC 73502-687-14
SOAANZ
60 mg
90 Tablets
SARFEZ
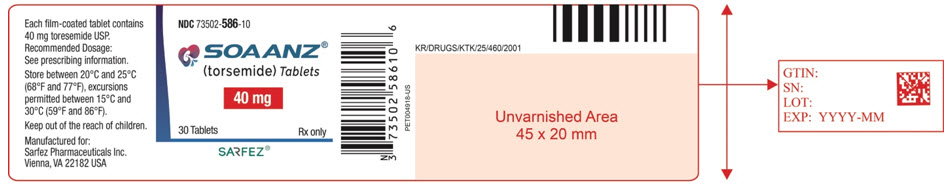
12PRINCIPAL DISPLAY PANEL - 40 mg Tablet Bottle Label - 586-10
NDC 73502-586-10
SOAANZ
40 mg
30 Tablets
SARFEZ
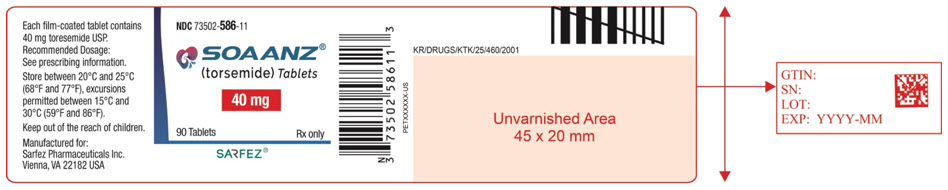
13PRINCIPAL DISPLAY PANEL - 40 mg Tablet Bottle Label - 586-11
NDC 73502-586-11
SOAANZ
40 mg
90 Tablets
SARFEZ
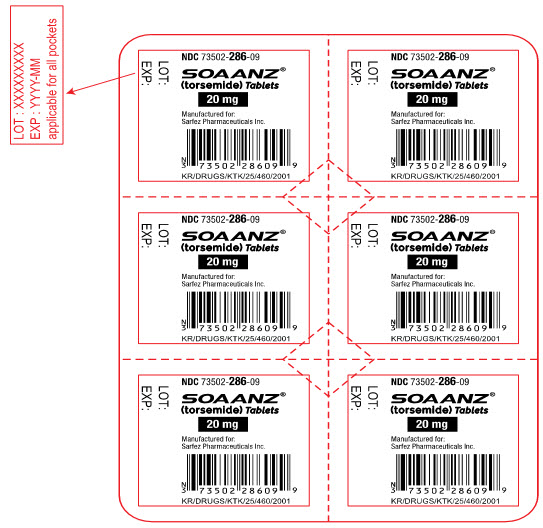
14PRINCIPAL DISPLAY PANEL - 20 mg Tablet Blister Pack Label
NDC 73502-286-09
SOAANZ
20 mg
Manufactured for:
KR/DRUGS/KTK/25/460/2001
LOT:
EXP:
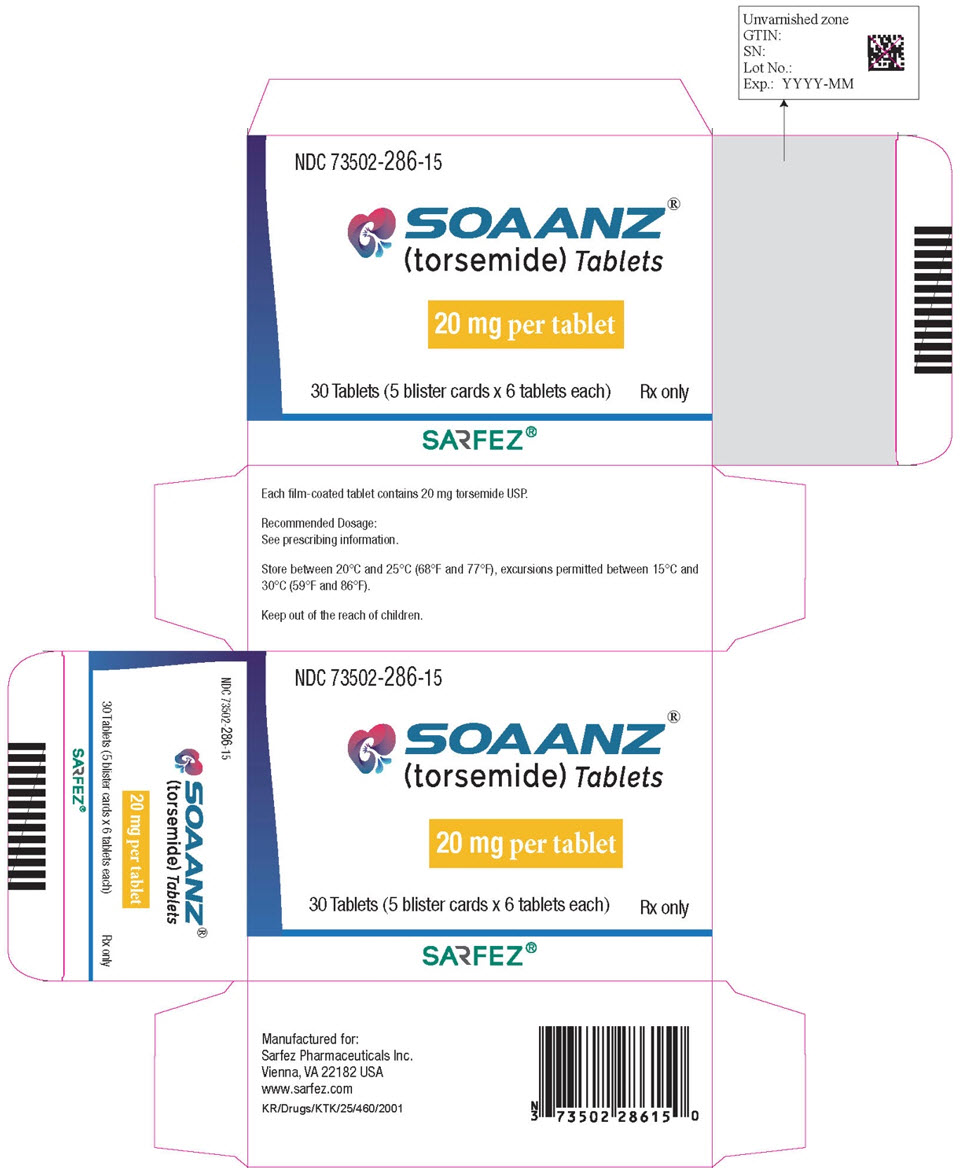
15PRINCIPAL DISPLAY PANEL - 20 mg Tablet Blister Pack Carton
NDC 73502-286-15
SOAANZ
20 mg per tablet
30 Tablets (5 blister cards x 6 tablets each)
Rx only
SARFEZ
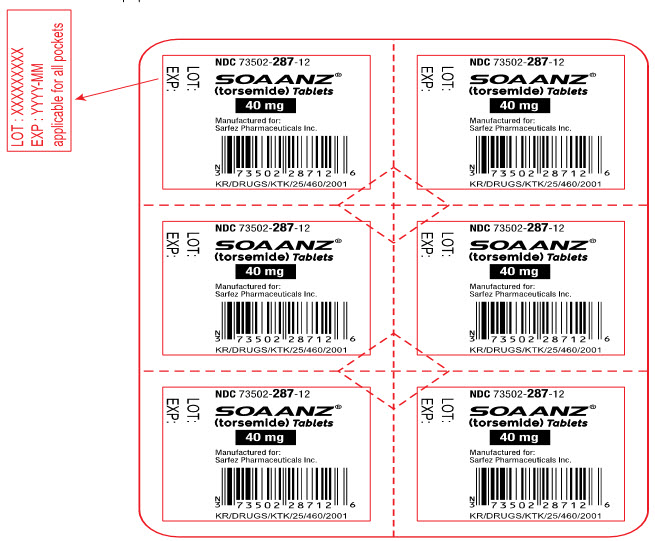
16PRINCIPAL DISPLAY PANEL - 40 mg Tablet Blister Pack Label
NDC 73502-287-12
SOAANZ
40 mg
Manufactured for:
KR/DRUGS/KTK/25/460/2001
LOT:
EXP:
17PRINCIPAL DISPLAY PANEL - 40 mg Tablet Blister Pack Carton
NDC 73502-287-16
SOAANZ
40 mg per tablet
30 Tablets (5 blister cards x 6 tablets each)
Rx only
SARFEZ

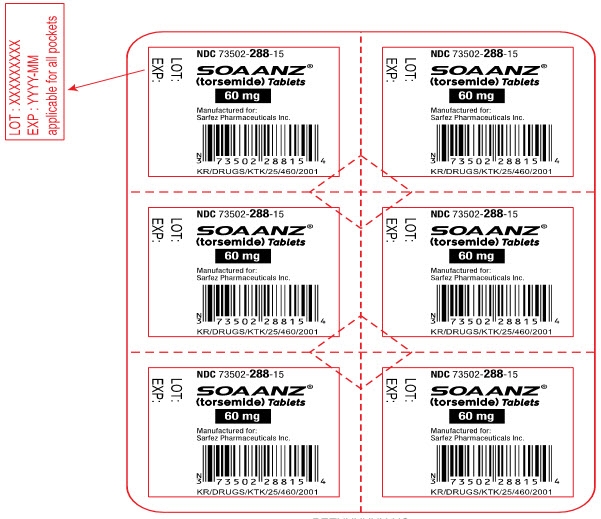
18PRINCIPAL DISPLAY PANEL - 60 mg Tablet Blister Pack Label
NDC 73502-288-15
SOAANZ
60 mg
Manufactured for:
KR/DRUGS/KTK/25/460/2001
LOT:
EXP:
19PRINCIPAL DISPLAY PANEL - 60 mg Tablet Blister Pack Carton
NDC 73502-288-17
SOAANZ
60 mg per tablet
30 Tablets (5 blister cards x 6 tablets each)
Rx only
SARFEZ
