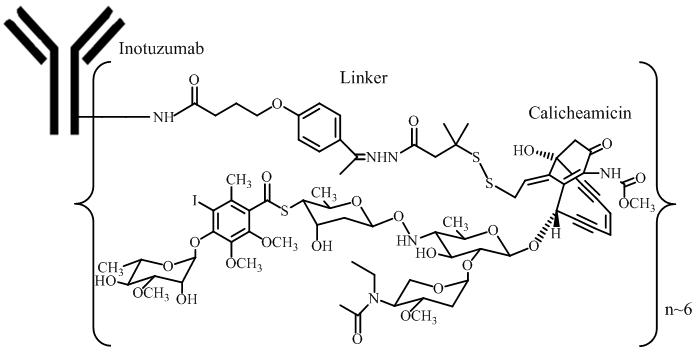
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Relapsed or Refractory B-cell Precursor ALL
Adult Patients
The safety of BESPONSA was evaluated in adult patients with relapsed or refractory B-cell precursor ALL in the INO-VATE ALL trial. The study was a randomized clinical study of BESPONSA (n=164) versus Investigator’s choice of chemotherapy (fludarabine + cytarabine + granulocyte colony-stimulating factor [FLAG], mitoxantrone + cytarabine [MXN/Ara-C], or high dose cytarabine [HIDAC]) (n=143)
Of the 164 patients who received BESPONSA, the median age was 47 years (range: 18–78 years), 56% were male, 68% had received 1 prior treatment regimen for ALL, 31% had received 2 prior treatment regimens for ALL, 68% were White, 19% were Asian, and 2% were Black.
In patients who received BESPONSA, the median duration of treatment was 8.9 weeks (range: 0.1–26.4 weeks), with a median of 3 treatment cycles started in each patient. In patients who received Investigator's choice of chemotherapy, the median duration of treatment was 0.9 weeks (range: 0.1–15.6 weeks), with a median of 1 treatment cycle started in each patient.
In patients who received BESPONSA, the most common (≥ 20%) adverse reactions were thrombocytopenia, neutropenia, infection, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, gamma-glutamyltransferase increased, and hyperbilirubinemia.
In patients who received BESPONSA, the most common (≥ 2%) serious adverse reactions were infection, febrile neutropenia, hemorrhage, abdominal pain, pyrexia, VOD, and fatigue.
In patients who received BESPONSA, the most common (≥ 2%) adverse reactions reported as the reason for permanent discontinuation were infection (6%), thrombocytopenia (2%), hyperbilirubinemia (2%), transaminases increased (2%), and hemorrhage (2%); the most common (≥ 5%) adverse reactions reported as the reason for dosing interruption were neutropenia (17%), infection (10%), thrombocytopenia (10%), transaminases increased (6%), and febrile neutropenia (5%); and the most common (≥ 1%) adverse reactions reported as the reason for dose reduction were neutropenia (1%), thrombocytopenia (1%), and transaminases increased (1%).
VOD was reported in 23/164 patients (14%) who received BESPONSA during or following treatment or following a HSCT after completion of treatment
Table 7 shows the adverse reactions with ≥ 10% incidence reported in patients with relapsed or refractory ALL who received BESPONSA or Investigator's choice of chemotherapy.
Additional adverse reactions (all grades) that were reported in less than 10% of patients treated with BESPONSA included: lipase increased (9%), abdominal distension (6%), amylase increased (5%), hyperuricemia (4%), ascites (4%), infusion related reaction (2%; includes the following: hypersensitivity and infusion related reaction), pancytopenia (2%; includes the following: bone marrow failure, febrile bone marrow aplasia, and pancytopenia), tumor lysis syndrome (2%), and electrocardiogram QT prolonged (1%).
Table 8 shows the clinically important laboratory abnormalities reported in patients with relapsed or refractory ALL who received BESPONSA or Investigator's choice of chemotherapy.
Pediatric Patients
The safety of BESPONSA in pediatric patients 1 year and older with relapsed or refractory CD22-positive B-cell precursor ALL was evaluated in a multicenter, single-arm, open-label study (ITCC-059)
Serious adverse reactions occurred in 62% of patients who received BESPONSA. Serious adverse reactions in > 2% of patients included infection (21%), febrile neutropenia (17%), VOD (15%), hemorrhage (4%), pyrexia (6%) and multiorgan failure (2%). Fatal adverse reactions occurred in 8% of patients who received BESPONSA, including multiorgan failure, lung infection, sepsis, and encephalopathy.
Permanent discontinuation of BESPONSA due to an adverse reaction occurred in 21% of patients. Adverse reactions which resulted in permanent discontinuation of BESPONSA in 2 or more patients included ALT increased and platelet count decreased.
Dosage interruptions of BESPONSA due to an adverse reaction occurred in 11% of patients. Adverse reactions which required dosage interruption of BESPONSA in 6 patients included increased transaminases, febrile neutropenia, and headache.
The most common adverse reactions (≥ 20%), including laboratory abnormalities, were thrombocytopenia, pyrexia, anemia, vomiting, infection, hemorrhage, neutropenia, nausea, leukopenia, febrile neutropenia, increased transaminases, abdominal pain, and headache.
Table 9 summarizes the adverse reactions in ITCC-059.
Table 10 summarizes select laboratory abnormalities in pediatric patients with CD22-positive relapsed/refractory ALL after receiving BESPONSA monotherapy in Study WI203581 (ITCC-059).