Aimovig
What is Aimovig (Erenumab-Aooe)?
Approved To Treat
Related Clinical Trials
Summary: The primary objective of this study is to estimate the proportion of major congenital malformations in infants of women with migraine exposed to erenumab-aooe during pregnancy compared to infants of women with migraine unexposed to erenumeb-aooe.
Summary: This is a placebo-controlled, multi-arm phase II platform screening trial designed to test the safety, pain responses, and pharmacodynamic activity of multiple experimental therapies simultaneously in participants with moderate-to-severe pain due to schwannomatosis (SWN). This Master Study is being conducted as a platform that may allow participants with pain associated with schwannomatosis to rec...
Summary: This study aims to assess the effect and safety of erenumab compared to placebo for the treatment of acute posttraumatic headache (PTH) in military service members and civilians with mild traumatic brain injury (mTBI).
Related Latest Advances
Brand Information
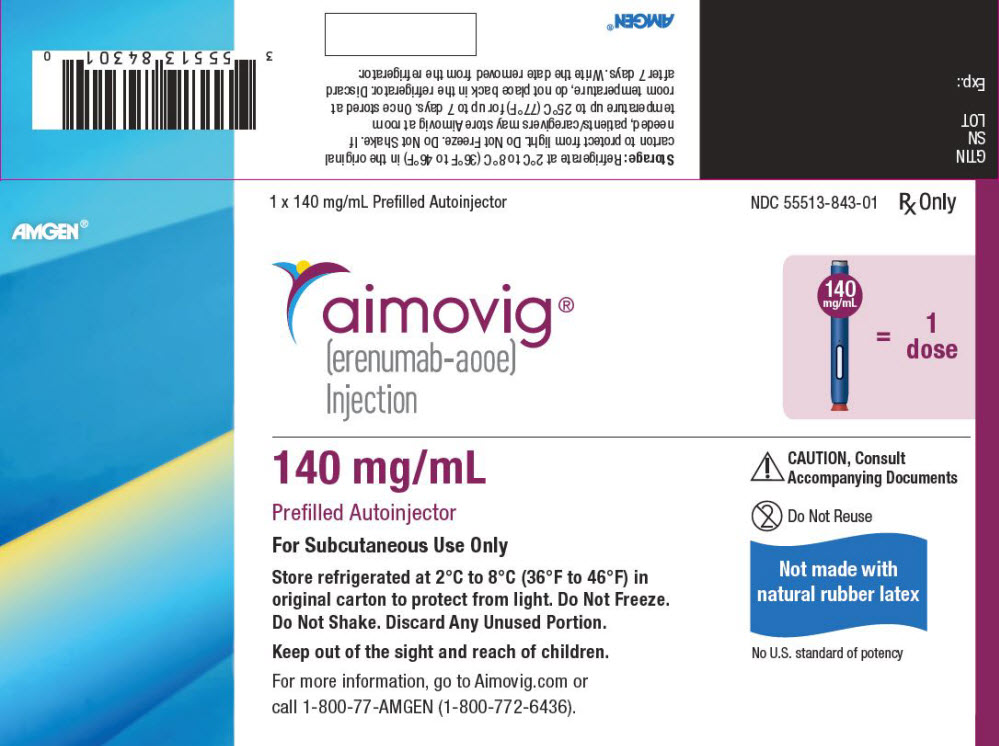
- Injection: 70 mg/mL in a single-dose prefilled SureClick
- Injection: 140 mg/mL in a single-dose prefilled SureClick
- Injection: 70 mg/mL in a single-dose prefilled syringe
- Injection: 140 mg/mL in a single-dose prefilled syringe
- Hypersensitivity Reactions
- Constipation with Serious Complications
- Hypertension
- Raynaud's Phenomenon

- Keep the autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the autoinjector in the original carton to protect it from light or physical damage.
- Do not freeze the autoinjector.
- Do not store the autoinjector in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.

- For example, when you are traveling, you may keep AIMOVIG at room temperature.
- It is okay to see air bubbles.
- Do not use the autoinjector if the medicine is cloudy, discolored, or contains flakes or particles.
- Do not use the autoinjector if the expiration date has passed.
- Do not use the autoinjector if:
- the white cap is missing or loose in carton.
- it has cracks or broken parts, or
- it has been dropped on a hard surface.
- AIMOVIG autoinjector (room temperature),
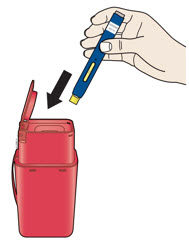
- Sharps disposal container [see
- Alcohol wipe,
- Adhesive bandage, and
- Cotton balls or gauze pads.
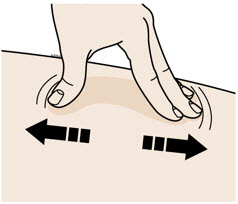
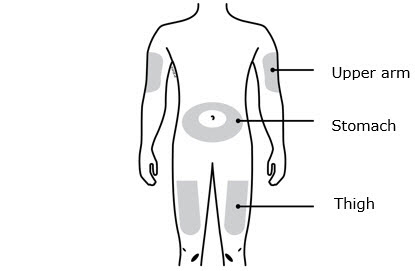
- Select the thigh or stomach (except for 2 inches around the belly button).
- Someone else can inject in your thigh, stomach, or back of the upper arm.

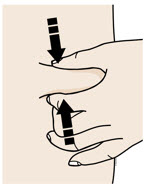
- Let the skin dry on its own.
- Do not touch this area again before injecting.
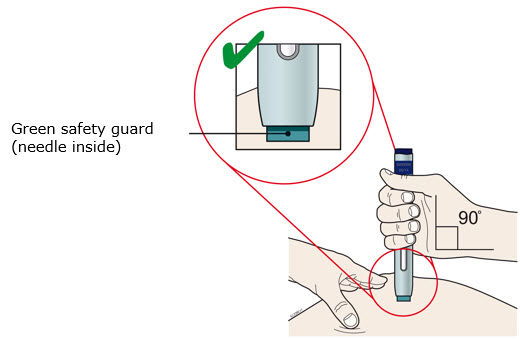
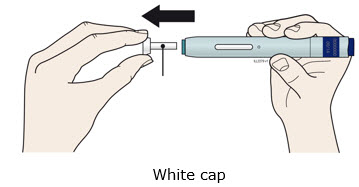
- Do not twist, bend, or wiggle the white cap to pull it off.
- Never put the white cap back on. It may damage the needle.
- Do not put your finger inside the dark green safety guard.
- It is normal to see a drop of medicine come out of the needle or dark green safety guard.
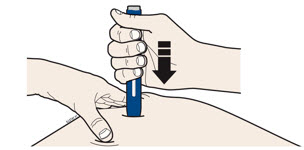
- Make sure you can see the window.
- Make sure the autoinjector is positioned straight on the injection site (at a 90-degree angle).
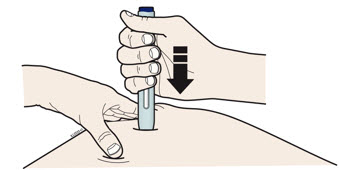
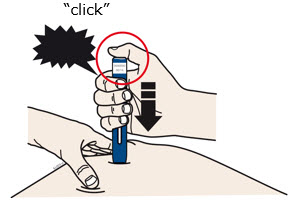
- The dark green safety guard pushes in and unlocks the purple start button.
- You may hear or feel a click.
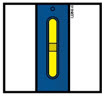
- The window starts to turn yellow.
- It is okay to let go of the purple start button.
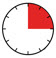
- The injection may take up to
- You may hear or feel a click.
- After the window turns fully yellow, lift the autoinjector away from the skin.
- The dark green safety guard locks around the needle.
- Do not touch the dark green safety guard.
- A small drop of liquid on the injection site is okay.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site.
- Apply an adhesive bandage if necessary.
- Do not reuse the autoinjector.
- Do not touch the dark green safety guard.
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- Keep the autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the autoinjector in the original carton to protect it from light or physical damage.
- Do not freeze the autoinjector.
- Do not store the autoinjector in extreme heat or cold. For example, avoid storing in your vehicle's glove box or trunk.
- For example, when you are traveling, you may keep AIMOVIG at room temperature.
- It is okay to see air bubbles.
- Do not use the autoinjector if the medicine is cloudy, discolored, or contains flakes or particles.
- Do not use the autoinjector if the expiration date has passed.
- Do not use the autoinjector if:
- the orange cap is missing or loose in carton.
- it has cracks or broken parts, or
- it has been dropped on a hard surface.
- AIMOVIG autoinjector (room temperature),
- Sharps disposal container [see
- Alcohol wipe,
- Adhesive bandage, and
- Cotton balls or gauze pads
- Select the thigh or stomach (except for 2 inches around the belly button).
- Someone else can inject in your thigh, stomach, or back of the upper arm.

- Let the skin dry on its own.
- Do not touch this area again before injecting.

- Do not twist, bend, or wiggle the orange cap to pull it off.
- Never put the orange cap back on. It may damage the needle.
- Do not put your finger inside the yellow safety guard.
- It is normal to see a drop of medicine come out of the needle or yellow safety guard.
- Make sure you can see the window.
- Make sure the autoinjector is positioned straight on the injection site (at a 90-degree angle).
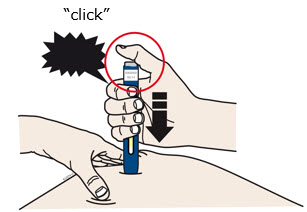
- The yellow safety guard pushes in and unlocks the gray start button.
- You may hear or feel a click.
- The window starts to turn yellow.
- It is okay to let go of the gray start button.
- The injection may take up to
- You may hear or feel a click.
- After the window turns fully yellow, lift the autoinjector away from the skin.
- The yellow safety guard locks around the needle.
- Do not touch the yellow safety guard.
- A small drop of liquid on the injection site is okay.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site.
- Apply an adhesive bandage if necessary.
- Do not reuse the autoinjector.
- Do not touch the yellow safety guard.
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.

- Keep the syringe out of the reach of children.
- Keep the syringe in the original carton to protect from light.
- The syringe should be kept in the refrigerator at 36°F to 46°F (2°C to 8°C).
- After removing AIMOVIG from the refrigerator, it can be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 7 days.
- Throw away AIMOVIG that has been left at room temperature for more than 7 days.
- Do not freeze.
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- Do not use a syringe after the expiration date on the label.
- Do not shake the syringe.
- Do not remove the gray needle cap from the syringe until you are ready to inject.
- Do not use the syringe if it has been frozen.
- Do not use a syringe if it has been dropped on a hard surface. Part of the syringe may be broken even if you cannot see the break. Use a new syringe, and call 1-800-77-AMGEN (1-800-772-6436).
- The prefilled syringe is not made with natural rubber latex.
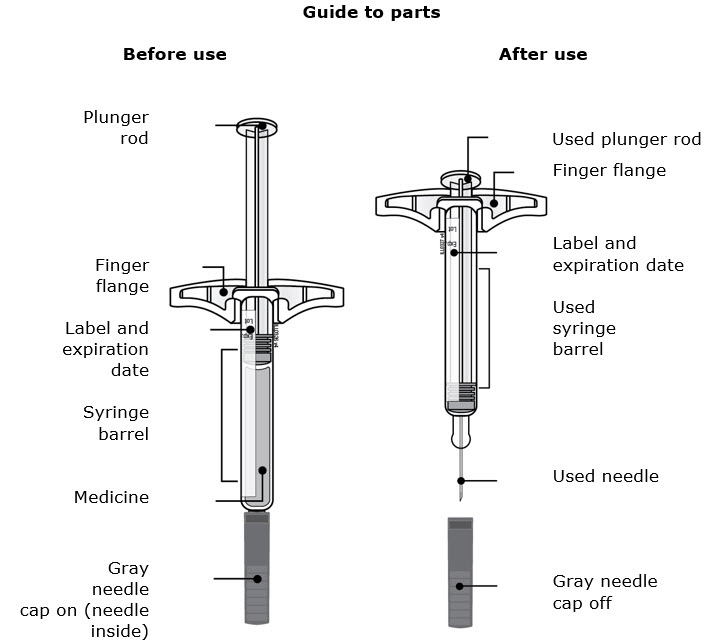
- Do not grab the plunger rod.
- Do not grab the gray needle cap.
- Do not remove the gray needle cap until you are ready to inject.
- Do not remove the finger flange. This is part of the syringe.
- Do not put the syringe back in the refrigerator after it has reached room temperature.
- Do not try to warm the syringe by using a heat source such as hot water or microwave.
- Do not leave the syringe in direct sunlight.
- Do not shake the syringe.

- Do not use the syringe if the medicine is cloudy or discolored or contains flakes or particles.
- Do not use the syringe if any part appears cracked or broken.
- Do not use the syringe if the syringe has been dropped.
- Do not use the syringe if the gray needle cap is missing or not securely attached.
- Do not use the syringe if the expiration date printed on the label has passed.
- New syringe
- Alcohol wipes
- Cotton balls or gauze pads
- Adhesive bandages
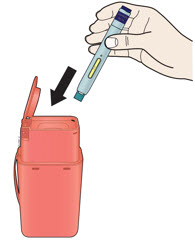
- Sharps disposal container

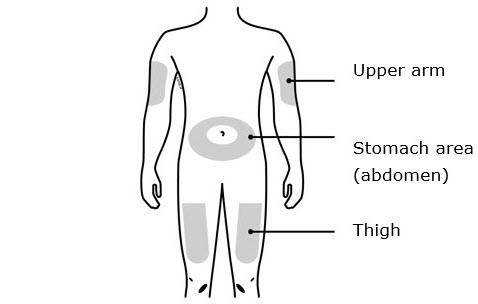
- Your thigh
- Stomach area (abdomen), except for a
- Outer area of upper arm (only if someone else is giving you the injection)
- Do not touch this area again before injecting.
- Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting directly into raised, thick, red, or scaly skin patch or lesion, or areas with scars or stretch marks.
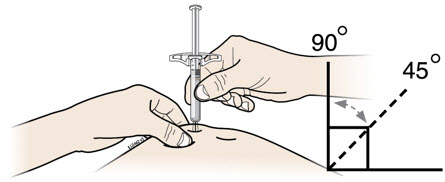
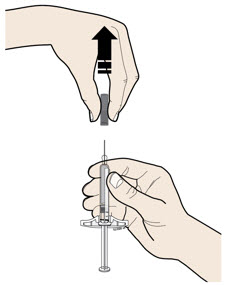
- Do not twist or bend the gray needle cap.
- Do not put the gray needle cap back onto the syringe.
- Do not remove the gray needle cap from the syringe until you are ready to inject.
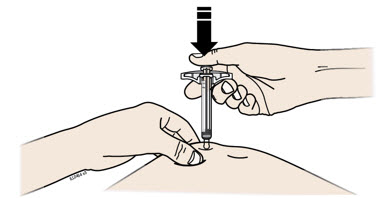
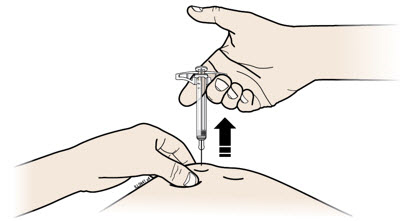
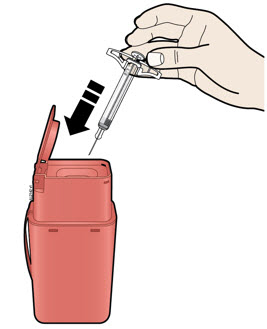

- Made of a heavy-duty plastic
- Can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- Upright and stable during use
- Leak-resistant
- Properly labeled to warn of hazardous waste inside the container
- Do not reuse the syringe.
- Do not recycle the syringe or sharps disposal container or throw them into household trash.